R-Ras Deficiency in Pericytes Causes Frequent Microphthalmia and Perturbs Retinal Vascular Development
- PMID: 33873190
- PMCID: PMC8263468
- DOI: 10.1159/000514555
R-Ras Deficiency in Pericytes Causes Frequent Microphthalmia and Perturbs Retinal Vascular Development
Abstract
Purpose: The retinal vasculature is heavily invested by pericytes. Small GTPase R-Ras is highly expressed in endothelial cells and pericytes, suggesting importance of this Ras homolog for the regulation of the blood vessel wall. We investigated the specific contribution of pericyte-expressed R-Ras to the development of the retinal vasculature.
Methods: The effect of R-Ras deficiency in pericytes was analyzed in pericyte-targeted conditional Rras knockout mice at birth and during the capillary plexus formation in the neonatal retina.
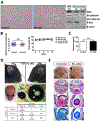
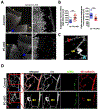
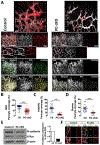
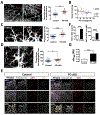
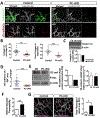
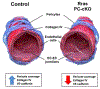
Results: The offspring of these mice frequently exhibited unilateral microphthalmia. Analyses of the developing retinal vasculature in the eyes without microphthalmia revealed excessive endothelial cell proliferation, sprouting, and branching of the capillary plexus in these animals. These vessels were structurally defective with diminished pericyte coverage and basement membrane formation. Furthermore, these vessels showed reduced VE-cadherin staining and significantly elevated plasma leakage indicating the breakdown of the blood-retinal barrier. This defect was associated with considerable macrophage infiltration in the retina.
Conclusions: The normal retinal vascular development is dependent on R-Ras expression in pericytes, and the absence of it leads to unattenuated angiogenesis and significantly weakens the blood-retinal barrier. Our findings underscore the importance of R-Ras for pericyte function during the normal eye development.
Keywords: Angiogenesis; Pericyte; R-Ras; Retina; Vascular permeability.
© 2021 S. Karger AG, Basel.
Conflict of interest statement
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Endothelial Cell-Specific Inactivation of TSPAN12 (Tetraspanin 12) Reveals Pathological Consequences of Barrier Defects in an Otherwise Intact Vasculature.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2691-2705. doi: 10.1161/ATVBAHA.118.311689. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354230 Free PMC article.
-
Therapeutic regulation of VE-cadherin with a novel oligonucleotide drug for diabetic eye complications using retinopathy mouse models.Diabetologia. 2019 Feb;62(2):322-334. doi: 10.1007/s00125-018-4770-4. Epub 2018 Nov 15. Diabetologia. 2019. PMID: 30443753
-
Pericytes promote selective vessel regression to regulate vascular patterning.Blood. 2012 Aug 16;120(7):1516-27. doi: 10.1182/blood-2011-01-332338. Epub 2012 Jun 27. Blood. 2012. PMID: 22740442
-
Pericyte-Endothelial Interactions in the Retinal Microvasculature.Int J Mol Sci. 2020 Oct 8;21(19):7413. doi: 10.3390/ijms21197413. Int J Mol Sci. 2020. PMID: 33049983 Free PMC article. Review.
-
Targeting pericyte retention in Diabetic Retinopathy: a review.Ann Med. 2024 Dec;56(1):2398200. doi: 10.1080/07853890.2024.2398200. Epub 2024 Sep 13. Ann Med. 2024. PMID: 39268600 Free PMC article. Review.
Cited by
-
Akt3 activation by R-Ras in an endothelial cell enforces quiescence and barrier stability of neighboring endothelial cells via Jagged1.Cell Rep. 2024 Mar 26;43(3):113837. doi: 10.1016/j.celrep.2024.113837. Epub 2024 Feb 24. Cell Rep. 2024. PMID: 38402584 Free PMC article.
-
Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR).Pharmaceutics. 2021 Nov 15;13(11):1932. doi: 10.3390/pharmaceutics13111932. Pharmaceutics. 2021. PMID: 34834347 Free PMC article.
-
Rho-Proteins and Downstream Pathways as Potential Targets in Sepsis and Septic Shock: What Have We Learned from Basic Research.Cells. 2021 Jul 21;10(8):1844. doi: 10.3390/cells10081844. Cells. 2021. PMID: 34440613 Free PMC article. Review.
-
Molecular Mechanisms Underlying Pathological and Therapeutic Roles of Pericytes in Atherosclerosis.Int J Mol Sci. 2022 Oct 1;23(19):11663. doi: 10.3390/ijms231911663. Int J Mol Sci. 2022. PMID: 36232962 Free PMC article. Review.
-
Proteomics Analysis of R-Ras Deficiency in Oxygen Induced Retinopathy.Int J Mol Sci. 2023 Apr 26;24(9):7914. doi: 10.3390/ijms24097914. Int J Mol Sci. 2023. PMID: 37175621 Free PMC article.
References
-
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9;685–693. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/Pericyte Interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

