Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease
- PMID: 33874858
- PMCID: PMC8078720
- DOI: 10.1080/19490976.2021.1907272
Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease
Abstract
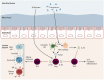
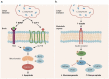
Clostridium butyricum is a butyrate-producing human gut symbiont that has been safely used as a probiotic for decades. C. butyricum strains have been investigated for potential protective or ameliorative effects in a wide range of human diseases, including gut-acquired infection, intestinal injury, irritable bowel syndrome, inflammatory bowel disease, neurodegenerative disease, metabolic disease, and colorectal cancer. In this review we summarize the studies on C. butyricum supplementation with special attention to proposed mechanisms for the associated health benefits and the supporting experimental evidence. These mechanisms center on molecular signals (especially butyrate) as well as immunological signals in the digestive system that cascade well beyond the gut to the liver, adipose tissue, brain, and more. The safety of probiotic C. butyricum strains appears well-established. We identify areas where additional human randomized controlled trials would provide valuable further data related to the strains' utility as an intervention.
Keywords: Clostridium butyricum; butyrate; cancer; immunity; inflammation; intestinal barrier; irritable bowel syndrome; metabolic disease; neurodegeneration; short chain fatty acid.
Conflict of interest statement
All authors are employees and stock/stock option shareholders of Pendulum Therapeutics, Inc (formerly known as ‘Whole Biome Inc.’). OK owns stock in GlySens, Inc, has stock options in ViaCyte, Inc, and is a consultant to NuSirt BioPharma, Circius, and NanoPrecision Medical.
Figures




Similar articles
-
Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota.Cancer Lett. 2020 Jan 28;469:456-467. doi: 10.1016/j.canlet.2019.11.019. Epub 2019 Nov 14. Cancer Lett. 2020. PMID: 31734354
-
Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells.World J Gastroenterol. 2019 Sep 28;25(36):5469-5482. doi: 10.3748/wjg.v25.i36.5469. World J Gastroenterol. 2019. PMID: 31576093 Free PMC article.
-
Remodeling gut microbiota by Clostridium butyricum (C.butyricum) attenuates intestinal injury in burned mice.Burns. 2020 Sep;46(6):1373-1380. doi: 10.1016/j.burns.2020.01.007. Epub 2020 Jan 31. Burns. 2020. PMID: 32014349
-
The Potential of Clostridium butyricum to Preserve Gut Health, and to Mitigate Non-AIDS Comorbidities in People Living with HIV.Probiotics Antimicrob Proteins. 2024 Aug;16(4):1465-1482. doi: 10.1007/s12602-024-10227-1. Epub 2024 Feb 9. Probiotics Antimicrob Proteins. 2024. PMID: 38336953 Review.
-
Clostridium butyricum, a future star in sepsis treatment.Front Cell Infect Microbiol. 2024 Dec 6;14:1484371. doi: 10.3389/fcimb.2024.1484371. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39711782 Free PMC article. Review.
Cited by
-
Caenorhabditis elegans as a Convenient Animal Model for Microbiome Studies.Int J Mol Sci. 2024 Jun 18;25(12):6670. doi: 10.3390/ijms25126670. Int J Mol Sci. 2024. PMID: 38928375 Free PMC article. Review.
-
The Role of Short-Chain Fatty Acids, Particularly Butyrate, in Oncological Immunotherapy with Checkpoint Inhibitors: The Effectiveness of Complementary Treatment with Clostridium butyricum 588.Microorganisms. 2024 Jun 19;12(6):1235. doi: 10.3390/microorganisms12061235. Microorganisms. 2024. PMID: 38930617 Free PMC article. Review.
-
Antibiotic-induced depletion of Clostridium species increases the risk of secondary fungal infections in preterm infants.Front Cell Infect Microbiol. 2022 Aug 31;12:981823. doi: 10.3389/fcimb.2022.981823. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36118040 Free PMC article.
-
Exploring substrate-microbe interactions: a metabiotic approach toward developing targeted synbiotic compositions.Gut Microbes. 2024 Jan-Dec;16(1):2305716. doi: 10.1080/19490976.2024.2305716. Epub 2024 Feb 1. Gut Microbes. 2024. PMID: 38300741 Free PMC article. Review.
-
Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D.Int J Mol Sci. 2023 Jan 30;24(3):2627. doi: 10.3390/ijms24032627. Int J Mol Sci. 2023. PMID: 36768946 Free PMC article. Review.
References
-
- Finegold SM, Sutter VL, Mathisen GE.. Normal indigenous intestinal flora. In: Hentges D, editor. Human intestinal microflora in health and disease. New York: Academic Press; 1983. p. 3–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
