Mitochondrial protein CMPK2 regulates IFN alpha-enhanced foam cell formation, potentially contributing to premature atherosclerosis in SLE
- PMID: 33874983
- PMCID: PMC8054390
- DOI: 10.1186/s13075-021-02470-6
Mitochondrial protein CMPK2 regulates IFN alpha-enhanced foam cell formation, potentially contributing to premature atherosclerosis in SLE
Abstract
Background: Premature atherosclerosis occurs in patients with SLE; however, the mechanisms remain unclear. Both mitochondrial machinery and proinflammatory cytokine interferon alpha (IFN-α) potentially contribute to atherogenic processes in SLE. Here, we explore the roles of the mitochondrial protein cytidine/uridine monophosphate kinase 2 (CMPK2) in IFN-α-mediated pro-atherogenic events.
Methods: Foam cell measurements were performed by oil red O staining, Dil-oxLDL uptake and the BODIPY approach. The mRNA and protein levels were measured by qPCR and Western blotting, respectively. Isolation of CD4+ T cells and monocytes was performed with monoclonal antibodies conjugated with microbeads. Manipulation of protein expression was conducted by either small interference RNA (siRNA) knockdown or CRISPR/Cas9 knockout. The expression of mitochondrial reactive oxygen species (mtROS) was determined by flow cytometry and confocal microscopy.
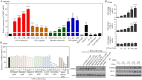
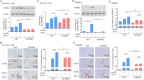
Results: IFN-α enhanced oxLDL-induced foam cell formation and Dil-oxLDL uptake by macrophages. In addition to IFN-α, several triggers of atherosclerosis, including thrombin and IFN-γ, can induce CMPK2 expression, which was elevated in CD4+ T cells and CD14+ monocytes isolated from SLE patients compared to those isolated from controls. The analysis of cellular subfractions revealed that CMPK2 was present in both mitochondrial and cytosolic fractions. IFN-α-induced CMPK2 expression was inhibited by Janus kinase (JAK)1/2 and tyrosine kinase 2 (Tyk2) inhibitors. Both the knockdown and knockout of CMPK2 attenuated IFN-α-mediated foam cell formation, which involved the reduction of scavenger receptor class A (SR-A) expression. CMPK2 also regulated IFN-α-enhanced mtROS production and inflammasome activation.
Conclusions: The study suggests that CMPK2 plays contributing roles in the pro-atherogenic effects of IFN-α.
Keywords: Atherosclerosis; CMPK2; Foam cell formation; Interferon alpha; Macrophages; Mitochondria.
Conflict of interest statement
The authors declare that they do not have competing interests.
Figures




References
-
- Ramirez GA, Efthymiou M, Isenberg DA, Cohen H. Under crossfire: thromboembolic risk in systemic lupus erythematosus. Rheumatology (Oxford). 2019;58:940–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

