Characterization and functional analysis of cathelicidin-MH, a novel frog-derived peptide with anti-septicemic properties
- PMID: 33875135
- PMCID: PMC8057816
- DOI: 10.7554/eLife.64411
Characterization and functional analysis of cathelicidin-MH, a novel frog-derived peptide with anti-septicemic properties
Abstract
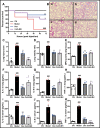
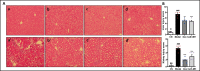
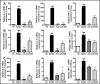
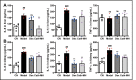
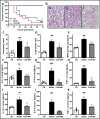
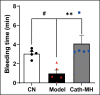
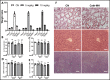
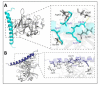
Antimicrobial peptides form part of the innate immune response and play a vital role in host defense against pathogens. Here we report a new antimicrobial peptide belonging to the cathelicidin family, cathelicidin-MH (cath-MH), from the skin of Microhyla heymonsivogt frog. Cath-MH has a single α-helical structure in membrane-mimetic environments and is antimicrobial against fungi and bacteria, especially Gram-negative bacteria. In contrast to other cathelicidins, cath-MH suppresses coagulation by affecting the enzymatic activities of tissue plasminogen activator, plasmin, β-tryptase, elastase, thrombin, and chymase. Cath-MH protects against lipopolysaccharide (LPS)- and cecal ligation and puncture-induced sepsis, effectively ameliorating multiorgan pathology and inflammatory cytokine through its antimicrobial, LPS-neutralizing, coagulation suppressing effects as well as suppression of MAPK signaling. Taken together, these data suggest that cath-MH is an attractive candidate therapeutic agent for the treatment of septic shock.
Keywords: antimicrobial peptides; cathelicidin; immunology; inflammation; microhyla heymonsivogt; mouse; sepsis.
© 2021, Chai et al.
Conflict of interest statement
JC, XC, TY, BZ, QZ, JW, BK, LM, TP, IS, MK, XX No competing interests declared
Figures








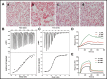
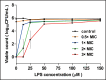
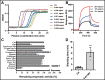
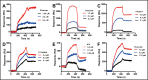

References
-
- Assumpção TC, Mizurini DM, Ma D, Monteiro RQ, Ahlstedt S, Reyes M, Kotsyfakis M, Mather TN, Andersen JF, Lukszo J, Ribeiro JMC, Francischetti IMB. Ixonnexin from tick saliva promotes fibrinolysis by interacting with plasminogen and Tissue-Type plasminogen activator, and prevents arterial thrombosis. Scientific Reports. 2018;8:4806. doi: 10.1038/s41598-018-22780-1. - DOI - PMC - PubMed
-
- Coorens M, Schneider VAF, de Groot AM, van Dijk A, Meijerink M, Wells JM, Scheenstra MR, Veldhuizen EJA, Haagsman HP. Cathelicidins inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner. The Journal of Immunology. 2017;199:1418–1428. doi: 10.4049/jimmunol.1602164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

