Respiratory mucus as a virus-host range determinant
- PMID: 33875348
- PMCID: PMC8503944
- DOI: 10.1016/j.tim.2021.03.014
Respiratory mucus as a virus-host range determinant
Abstract
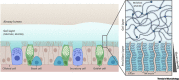
Efficient penetration of the mucus layer is needed for respiratory viruses to avoid mucociliary clearance prior to infection. Many respiratory viruses bind to glycans on the heavily glycosylated mucins that give mucus its gel-like characteristics. Influenza viruses, some paramyxoviruses, and coronaviruses avoid becoming trapped in the mucus by releasing themselves by means of their envelope-embedded enzymes that destroy glycan receptors. For efficient infection, receptor binding and destruction need to be in balance with the host receptor repertoire. Establishment in a novel host species requires resetting of the balance to adapt to the different glycan repertoire encountered. Growing understanding of species-specific mucosal glycosylation patterns and the dynamic interaction with respiratory viruses identifies the mucus layer as a major host-range determinant and barrier for zoonotic transfer.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
There are no interests to declare.
Figures
References
-
- Matrosovich M., et al. In: SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans. Gerardy-Schahn R., et al., editors. Springer International Publishing; 2015. Sialic acid receptors of viruses; pp. 1–28.
-
- Varki A., et al. In: Essentials of Glycobiology. Varki A., et al., editors. Cold Spring Harbor Laboratory Press; 2017. Sialic acids and other nonulosonic acids.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

