New Perspectives on Antimicrobial Agents: Ceftolozane-Tazobactam
- PMID: 33875428
- PMCID: PMC8218675
- DOI: 10.1128/AAC.02318-20
New Perspectives on Antimicrobial Agents: Ceftolozane-Tazobactam
Abstract
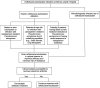
Ceftolozane-tazobactam (C/T) is a new fifth-generation cephalosporin/beta-lactamase inhibitor combination approved by the Food and Drug Administration and the European Medicines Agency for treatment of complicated intraabdominal infections, complicated urinary tract infections, and hospital-acquired pneumonia in adult patients. This review will briefly describe the pharmacology of C/T and focus on the emerging clinical trial and real-world data supporting its current utilization. Additionally, our synthesis of these data over time has set our current usage of C/T at Barnes-Jewish Hospital (BJH). C/T is primarily employed as directed monotherapy at BJH when Pseudomonas aeruginosa isolates are identified with resistance to other beta-lactams. C/T can also be used empirically in specific clinical situations at BJH prior to microbiological detection of an antibiotic-resistant P. aeruginosa isolate. These situations include critically ill patients in the intensive care unit (ICU) setting, where there is a high likelihood of infection with multidrug-resistant (MDR) P. aeruginosa; patients failing therapy with a carbapenem; specific patient populations known to be at high risk for infection with MDR P. aeruginosa (e.g., lung transplant and cystic fibrosis patients); and patients know to have previous infection or colonization with MDR P. aeruginosa.
Keywords: antimicrobial resistance.
Figures
References
-
- Shortridge D, Pfaller MA, Castanheira M, Flamm RK. 2018. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobactericeae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2013–2016) as part of the surveillance program: program to assess ceftolozane-tazobactam susceptibility. Microb Drug Resist 24:563–577. doi:10.1089/mdr.2017.0266. - DOI - PubMed
-
- Walkty A, Adam H, Baxter M, Lagace-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. 2018. In vitro activity of ceftolozane/tazobactam versus antimicrobial non-susceptible Pseudomonas aeruginosa clinical isolates including MDR and XDR isolates obtained from across Canada as part of the CANWARD study, 2008–16. J Antimicrob Chemother 73:703–708. doi:10.1093/jac/dkx468. - DOI - PubMed
-
- Castanheira M, Johnson MG, Yu B, Huntington JA, Carmelitano P, Bruno C, Rhee EG, Motyl M. 2020. Molecular characterization of baseline Enterobacterales and Pseudomonas aeruginosa isolates from a phase 3 nosocomial pneumonia (ASPECT-NP) clinical trial. Antimicrob Agents Chemother 65:e02461-20. doi:10.1128/AAC.02461-20. - DOI - PMC - PubMed
-
- Kuo SC, Liu CE, Lu PL, Chen YS, Lu MC, Ko WC, Hsueh PR, Chuang YC, Wang FD, SMART Asia-Pacific Group . 2020. Activity of ceftolozane-tazobactam against Gram-negative pathogens isolated from lower respiratory tract infections in the Asia-Pacific region: SMART 2015–2016. Int J Antimicrob Agents 55:105883. doi:10.1016/j.ijantimicag.2020.105883. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources