Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo
- PMID: 33875437
- PMCID: PMC8373240
- DOI: 10.1128/AAC.00013-21
Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo
Abstract
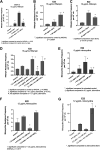
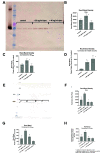
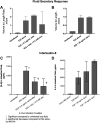
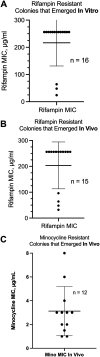
The SOS response to DNA damage is a conserved stress response in Gram-negative and Gram-positive bacteria. Although this pathway has been studied for years, its relevance is still not familiar to many working in the fields of clinical antibiotic resistance and stewardship. Under some conditions, the SOS response favors DNA repair and preserves the genetic integrity of the organism. On the other hand, the SOS response also includes induction of error-prone DNA polymerases, which can increase the rate of mutation, called the mutator phenotype or "hypermutation." As a result, mutations can occur in genes conferring antibiotic resistance, increasing the acquisition of resistance to antibiotics. Almost all of the work on the SOS response has been on bacteria exposed to stressors in vitro. In this study, we sought to quantitate the effects of SOS-inducing drugs in vivo, in comparison with the same drugs in vitro. We used a rabbit model of intestinal infection with enteropathogenic Escherichia coli strain E22. SOS-inducing drugs triggered the mutator phenotype response in vivo as well as in vitro. Exposure of E. coli strain E22 to ciprofloxacin or zidovudine, both of which induce the SOS response in vitro, resulted in increased antibiotic resistance to 3 antibiotics: rifampin, minocycline, and fosfomycin. Zinc was able to inhibit the SOS-induced emergence of antibiotic resistance in vivo, as previously observed in vitro. Our findings may have relevance in reducing the emergence of resistance to new antimicrobial drugs.
Keywords: RecA; enteropathogenic E. coli; fosfomycin; heteroresistance; hypermutation; minocycline; rabbit model; rifampin; zidovudine; zinc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

