Effectiveness of HPV vaccine by age at vaccination and number of doses: protocol for a population-based matched case-control study
- PMID: 33875441
- PMCID: PMC8057558
- DOI: 10.1136/bmjopen-2020-043093
Effectiveness of HPV vaccine by age at vaccination and number of doses: protocol for a population-based matched case-control study
Abstract
Introduction: In 2006, the first human papillomavirus (HPV) vaccine was approved by the Food and Drug Administration in the USA based on pre-licensure clinical trials that found it to be highly efficacious at preventing persistent infection and precancerous, high-grade cervical lesions (HGCLs) caused by viral types the vaccine protects against. However, the real-world effectiveness of HPV vaccines as used in clinical practice may be quite different from the efficacy found in pre-licensure clinical trials. More than 10 years have passed since the introduction of the vaccine programme. It is critical to determine if the full benefits of HPV are being realised in real-world settings.
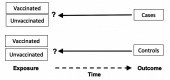
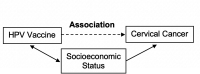
Methods and analysis: The objectives of this study were to estimate the effectiveness of HPV vaccines as used in real-world clinical settings and to determine the degree to which the vaccine's effectiveness varies based on age at the time of immunisation and the number of doses received. The study will be a population-based, matched case-control study. Cases will be women with newly diagnosed HGCL associated with HPV types 16 and 18. Matched controls will be women with a normal Pap test result, matched individually to cases in a 2:1 ratio by age, a practice and date of testing. Medical records will be reviewed to determine dates of receipt of the HPV vaccine for all participants. We will use multivariate conditional logistic regression to control for potential confounders.
Ethics and dissemination: This protocol presents minimal risk to the subjects. This protocol has received approval from the Institutional Review Board of Yale University (HIC: 1502015308), and a Health Insurance Portability and Accountability Act (HIPAA) Waiver of Authorisation has been granted to allow investigators to recruit subjects for the study. Findings will be disseminated through peer-reviewed, open-access scientific journals and conference presentations.
Keywords: epidemiology; gynaecological oncology; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Dr Niccolai has served as Scientific Advisor for Merck. Dr Sheth receives Gardasil 9 from Merck at no cost for research and has served as a consultant for Merck. All other authors declare no conflicts of interest.
Figures
References
-
- Administration USFaD . FDA licenses new vaccine for prevention of cervical cancer and other diseases caused by human papillomavirus, 2006.
-
- Administration USFaD . FDA approves new vaccine for prevention of cervical cancer, 2009.
-
- Administration USFaD . FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials