Switching to Once-Weekly Insulin Icodec Versus Once-Daily Insulin Glargine U100 in Type 2 Diabetes Inadequately Controlled on Daily Basal Insulin: A Phase 2 Randomized Controlled Trial
- PMID: 33875485
- PMCID: PMC8323191
- DOI: 10.2337/dc20-2877
Switching to Once-Weekly Insulin Icodec Versus Once-Daily Insulin Glargine U100 in Type 2 Diabetes Inadequately Controlled on Daily Basal Insulin: A Phase 2 Randomized Controlled Trial
Abstract
Objective: Insulin icodec (icodec) is a novel once-weekly basal insulin analog. This trial investigated two approaches for switching to icodec versus once-daily insulin glargine 100 units/mL (IGlar U100) in people with type 2 diabetes receiving daily basal insulin and one or more oral glucose-lowering medications.
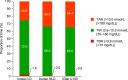
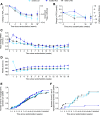
Research design and methods: This multicenter, open-label, treat-to-target phase 2 trial randomized (1:1:1) eligible basal insulin-treated (total daily dose 10-50 units) people with type 2 diabetes (HbA1c 7.0-10.0% [53.0-85.8 mmol/mol]) to icodec with an initial 100% loading dose (in which only the first dose was doubled [icodec LD]), icodec with no loading dose (icodec NLD), or IGlar U100 for 16 weeks. Primary end point was percent time in range (TIR; 3.9-10.0 mmol/L [70-180 mg/dL]) during weeks 15 and 16, measured using continuous glucose monitoring. Key secondary end points included HbA1c, adverse events (AEs), and hypoglycemia.
Results: Estimated mean TIR during weeks 15 and 16 was 72.9% (icodec LD; n = 54), 66.0% (icodec NLD; n = 50), and 65.0% (IGlar U100; n = 50), with a statistically significant difference favoring icodec LD versus IGlar U100 (7.9%-points [95% CI 1.8-13.9]). Mean HbA1c reduced from 7.9% (62.8 mmol/mol) at baseline to 7.1% (54.4 mmol/mol icodec LD) and 7.4% (57.6 mmol/mol icodec NLD and IGlar U100); incidences and rates of AEs and hypoglycemic episodes were comparable.
Conclusions: Switching from daily basal insulin to once-weekly icodec was well tolerated and provided effective glycemic control. Loading dose use when switching to once-weekly icodec significantly increased percent TIR during weeks 15 and 16 versus once-daily IGlar U100, without increasing hypoglycemia risk.
Trial registration: ClinicalTrials.gov NCT03922750.
© 2021 by the American Diabetes Association.
Figures
Comment in
-
Weekly Insulin Becoming a Reality.Diabetes Care. 2021 Jul;44(7):1459-1461. doi: 10.2337/dci21-0011. Epub 2021 Jun 21. Diabetes Care. 2021. PMID: 34155035 No abstract available.
References
-
- American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020 [published correction appears in Diabetes Care 2020;43:1979]. Diabetes Care 2020;43(Suppl. 1):S98–S110 - PubMed
-
- Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin 2011;27(Suppl. 3):13–20 - PubMed
-
- Escalada J, Orozco-Beltran D, Morillas C, et al. . Attitudes towards insulin initiation in type 2 diabetes patients among healthcare providers: a survey research. Diabetes Res Clin Pract 2016;122:46–53 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

