Pentraxin-3-mediated complement activation in a swine model of renal ischemia/reperfusion injury
- PMID: 33875620
- PMCID: PMC8109140
- DOI: 10.18632/aging.202992
Pentraxin-3-mediated complement activation in a swine model of renal ischemia/reperfusion injury
Abstract
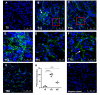
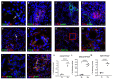
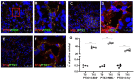
Pentraxins are a family of evolutionarily conserved pattern recognition molecules with pivotal roles in innate immunity and inflammation, such as opsonization of pathogens during bacterial and viral infections. In particular, the long Pentraxin 3 (PTX3) has been shown to regulate several aspects of vascular and tissue inflammation during solid organ transplantation. Our study investigated the role of PTX3 as possible modulator of Complement activation in a swine model of renal ischemia/reperfusion (I/R) injury. We demonstrated that I/R injury induced early PTX3 deposits at peritubular and glomerular capillary levels. Confocal laser scanning microscopy revealed PTX3 deposits co-localizing with CD31+ endothelial cells. In addition, PTX3 was associated with infiltrating macrophages (CD163), dendritic cells (SWC3a) and myofibroblasts (FSP1). In particular, we demonstrated a significant PTX3-mediated activation of classical (C1q-mediated) and lectin (MBL-mediated) pathways of Complement. Interestingly, PTX3 deposits co-localized with activation of the terminal Complement complex (C5b-9) on endothelial cells, indicating that PTX3-mediated Complement activation occurred mainly at the renal vascular level. In conclusion, these data indicate that PTX3 might be a potential therapeutic target to prevent Complement-induced I/R injury.
Keywords: classical pathway; complement system; ischemia/reperfusion injury; kidney; pentraxin 3.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

