Short-range quorum sensing controls horizontal gene transfer at micron scale in bacterial communities
- PMID: 33875666
- PMCID: PMC8055654
- DOI: 10.1038/s41467-021-22649-4
Short-range quorum sensing controls horizontal gene transfer at micron scale in bacterial communities
Abstract
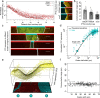
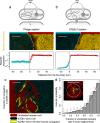
In bacterial communities, cells often communicate by the release and detection of small diffusible molecules, a process termed quorum-sensing. Signal molecules are thought to broadly diffuse in space; however, they often regulate traits such as conjugative transfer that strictly depend on the local community composition. This raises the question how nearby cells within the community can be detected. Here, we compare the range of communication of different quorum-sensing systems. While some systems support long-range communication, we show that others support a form of highly localized communication. In these systems, signal molecules propagate no more than a few microns away from signaling cells, due to the irreversible uptake of the signal molecules from the environment. This enables cells to accurately detect micron scale changes in the community composition. Several mobile genetic elements, including conjugative elements and phages, employ short-range communication to assess the fraction of susceptible host cells in their vicinity and adaptively trigger horizontal gene transfer in response. Our results underscore the complex spatial biology of bacteria, which can communicate and interact at widely different spatial scales.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Regulation of conjugative transfer of plasmids and integrative conjugative elements.Plasmid. 2017 May;91:82-89. doi: 10.1016/j.plasmid.2017.04.002. Epub 2017 Apr 22. Plasmid. 2017. PMID: 28438469 Review.
-
Social behaviours by Bacillus subtilis: quorum sensing, kin discrimination and beyond.Mol Microbiol. 2018 Dec;110(6):863-878. doi: 10.1111/mmi.14127. Epub 2018 Nov 1. Mol Microbiol. 2018. PMID: 30218468 Free PMC article. Review.
-
Self-sensing in Bacillus subtilis quorum-sensing systems.Nat Microbiol. 2018 Jan;3(1):83-89. doi: 10.1038/s41564-017-0044-z. Epub 2017 Oct 16. Nat Microbiol. 2018. PMID: 29038467 Free PMC article.
-
Identification, characterization and benefits of an exclusion system in an integrative and conjugative element of Bacillus subtilis.Mol Microbiol. 2019 Oct;112(4):1066-1082. doi: 10.1111/mmi.14359. Epub 2019 Aug 16. Mol Microbiol. 2019. PMID: 31361051 Free PMC article.
-
The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution.Philos Trans R Soc Lond B Biol Sci. 2007 Jul 29;362(1483):1185-93. doi: 10.1098/rstb.2007.2043. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17360276 Free PMC article. Review.
Cited by
-
Dormant phages communicate via arbitrium to control exit from lysogeny.Nat Microbiol. 2022 Jan;7(1):145-153. doi: 10.1038/s41564-021-01008-5. Epub 2021 Dec 9. Nat Microbiol. 2022. PMID: 34887546
-
Disentangling the feedback loops driving spatial patterning in microbial communities.NPJ Biofilms Microbiomes. 2025 Feb 20;11(1):32. doi: 10.1038/s41522-025-00666-1. NPJ Biofilms Microbiomes. 2025. PMID: 39979272 Free PMC article. Review.
-
Achieving spatially precise diagnosis and therapy in the mammalian gut using synthetic microbial gene circuits.Front Bioeng Biotechnol. 2022 Sep 2;10:959441. doi: 10.3389/fbioe.2022.959441. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36118573 Free PMC article. Review.
-
How bacteria actively use passive physics to make biofilms.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2403842121. doi: 10.1073/pnas.2403842121. Epub 2024 Sep 12. Proc Natl Acad Sci U S A. 2024. PMID: 39264745 Free PMC article.
-
Plasmid co-infection: linking biological mechanisms to ecological and evolutionary dynamics.Philos Trans R Soc Lond B Biol Sci. 2022 Jan 17;377(1842):20200478. doi: 10.1098/rstb.2020.0478. Epub 2021 Nov 29. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 34839701 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

