The adipokine orosomucoid alleviates adipose tissue fibrosis via the AMPK pathway
- PMID: 33875797
- PMCID: PMC8792011
- DOI: 10.1038/s41401-021-00666-9
The adipokine orosomucoid alleviates adipose tissue fibrosis via the AMPK pathway
Abstract
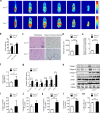
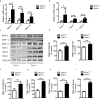
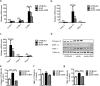
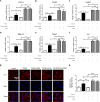
The excess deposition of underlying extracellular matrix (ECM) in adipose tissue is defined as adipose tissue fibrosis that is a major contributor to metabolic disorder such as obesity and type 2 diabetes. Anti-fibrosis therapy has received much attention in the treatment of metabolic disorders. Orosomucoid (ORM) is an acute-phase protein mainly produced by liver, which is also an adipokine. In this study, we investigated the effects of ORM on adipose tissue fibrosis and the potential mechanisms. We showed that ORM1-deficient mice exhibited an obese phenotype, manifested by excessive collagen deposition in adipose tissues and elevated expression of ECM regulators such as metalloproteinases (MMP-2, MMP-13, MMP-14) and tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2, TIMP-3). Administration of exogenous ORM (50 mg· kg-1· d-1, ip) for 7 consecutive days in high-fat diet (HFD)-fed mice and leptin receptor (LepR)-deficient db/db mice attenuated these abnormal expressions. Meanwhile, ORM administration stimulated AMP-activated protein kinase (AMPK) phosphorylation and decreased transforming growth factor-β1 (TGF-β1) level in adipose tissues of the mice. In TGF-β1-treated 3T3-L1 fibroblasts, ORM (10 μg/mL) improved the impaired expression profiles of fibrosis-related genes, whereas a selective AMPK inhibitor dorsomorphin (1 μmol/mL) abolished these effects. Together, our results suggest that ORM exerts a direct anti-fibrosis effect in adipose tissue via AMPK activation. ORM is expected to become a novel target for the treatment of adipose tissue fibrosis.
Keywords: 3T3-L1 fibroblasts; AMPK; ORM; TGF-β1; adipose tissue; dorsomorphin; fibrosis; metabolic disorders; obesity.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice.Biomed Pharmacother. 2018 Sep;105:121-129. doi: 10.1016/j.biopha.2018.05.110. Epub 2018 May 28. Biomed Pharmacother. 2018. PMID: 29852389
-
AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity.Diabetes. 2016 Aug;65(8):2295-310. doi: 10.2337/db15-1122. Epub 2016 May 13. Diabetes. 2016. PMID: 27207538 Free PMC article.
-
The Acute-Phase Protein Orosomucoid Regulates Food Intake and Energy Homeostasis via Leptin Receptor Signaling Pathway.Diabetes. 2016 Jun;65(6):1630-41. doi: 10.2337/db15-1193. Epub 2016 Mar 23. Diabetes. 2016. PMID: 27207522
-
Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice.Phytomedicine. 2016 Feb 15;23(2):181-90. doi: 10.1016/j.phymed.2015.12.018. Epub 2016 Jan 21. Phytomedicine. 2016. PMID: 26926180
-
Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation.J Biol Chem. 2010 Jul 16;285(29):22174-85. doi: 10.1074/jbc.M109.085464. Epub 2010 May 4. J Biol Chem. 2010. PMID: 20442402 Free PMC article.
Cited by
-
Friend or foe for obesity: How hepatokines remodel adipose tissues and translational perspective.Genes Dis. 2022 Feb 2;10(3):825-847. doi: 10.1016/j.gendis.2021.12.011. eCollection 2023 May. Genes Dis. 2022. PMID: 37396511 Free PMC article. Review.
-
More than carriers, orosomucoids are key metabolic modulators.Trends Endocrinol Metab. 2025 Jun;36(6):507-510. doi: 10.1016/j.tem.2024.11.015. Epub 2024 Dec 18. Trends Endocrinol Metab. 2025. PMID: 39701917 Review.
-
Urine Proteome in Distinguishing Hepatic Steatosis in Patients with Metabolic-Associated Fatty Liver Disease.Diagnostics (Basel). 2022 Jun 7;12(6):1412. doi: 10.3390/diagnostics12061412. Diagnostics (Basel). 2022. PMID: 35741222 Free PMC article.
-
Repurposing of Antiplatelet Agent: Cilostazol for the Treatment of Alcohol-Related Liver Disease.Gut Liver. 2025 May 15;19(3):318-326. doi: 10.5009/gnl240295. Epub 2025 Jan 8. Gut Liver. 2025. PMID: 39774120 Free PMC article. Review.
-
Effect of adipokines on bone marrow mesenchymal stem cell function.World J Stem Cells. 2025 May 26;17(5):106150. doi: 10.4252/wjsc.v17.i5.106150. World J Stem Cells. 2025. PMID: 40503369 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

