Endocrine toxicities of immune checkpoint inhibitors
- PMID: 33875857
- PMCID: PMC8769055
- DOI: 10.1038/s41574-021-00484-3
Endocrine toxicities of immune checkpoint inhibitors
Abstract
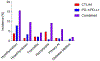
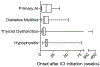
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target two key signalling pathways related to T cell activation and exhaustion, by binding to and inhibiting cytotoxic T lymphocyte antigen 4 (CTLA4) or PD1 and its ligand PDL1. ICIs, such as nivolumab, pembrolizumab and ipilimumab, are approved for the treatment of numerous and diverse cancer types, in various combination regimens, and are now an established cornerstone of cancer therapeutics. Toxicities induced by ICIs are autoimmune in nature and are referred to as immune-related adverse events (irAEs); these events can affect any organ system in an unpredictable fashion. Importantly, irAEs can manifest as endocrinopathies involving the thyroid (hypothyroidism or thyrotoxicosis), pituitary (hypophysitis), adrenal glands (adrenal insufficiency) and pancreas (diabetes mellitus). These events are a frequent source of acute and persistent morbidity in patients treated with ICIs and can even be fatal. Over the past few years, there has been a growing understanding of the underlying pathogenesis of irAEs that has led to the development of more effective management strategies. Herein, we review the current understanding of the pathobiology, clinical manifestations and treatment approaches to endocrine toxicities arising from ICIs.
Figures
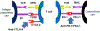
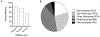
References
-
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. The New England journal of medicine 2018;379:2342–50. - PubMed
-
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine 2017;377:1824–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

