Neutralizing monoclonal antibodies for treatment of COVID-19
- PMID: 33875867
- PMCID: PMC8054133
- DOI: 10.1038/s41577-021-00542-x
Neutralizing monoclonal antibodies for treatment of COVID-19
Abstract
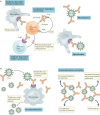
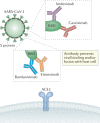
Several neutralizing monoclonal antibodies (mAbs) to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed and are now under evaluation in clinical trials. With the US Food and Drug Administration recently granting emergency use authorizations for neutralizing mAbs in non-hospitalized patients with mild-to-moderate COVID-19, there is an urgent need to discuss the broader potential of these novel therapies and to develop strategies to deploy them effectively in clinical practice, given limited initial availability. Here, we review the precedent for passive immunization and lessons learned from using antibody therapies for viral infections such as respiratory syncytial virus, Ebola virus and SARS-CoV infections. We then focus on the deployment of convalescent plasma and neutralizing mAbs for treatment of SARS-CoV-2. We review specific clinical questions, including the rationale for stratification of patients, potential biomarkers, known risk factors and temporal considerations for optimal clinical use. To answer these questions, there is a need to understand factors such as the kinetics of viral load and its correlation with clinical outcomes, endogenous antibody responses, pharmacokinetic properties of neutralizing mAbs and the potential benefit of combining antibodies to defend against emerging viral variants.
Conflict of interest statement
P.C.T. has received research grants, consultation fees and/or speaking fees from AbbVie, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Fresenius, Galapagos, Gilead, GlaxoSmithKline, Janssen, Eli Lilly and Company, Sanofi, Nordic Pharma, Pfizer, Roche and UCB. A.C.A., I.d.l.T. and M.M.H. are employees and shareholders of Eli Lilly and Company. K.W. has received research grants from Bristol-Myers Squibb and Pfizer and consulting fees from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Galapagos, GlaxoSmithKline, Gilead, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB. R.L.G. reports non-financial support from Gilead Sciences Inc. and personal fees from Gilead Sciences Inc. outside the submitted work.
Figures

References
-
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. - PubMed
-
- Regeneron Pharmaceuticals Inc. Fact sheet for health care providers: emergency use authorization (EUA) of casirivimab and imdevimab. Regeneronhttps://www.regeneron.com/sites/default/files/treatment-covid19-eua-fact... (2020).
-
- US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab. FDAhttps://www.fda.gov/media/143603/download (2020).
-
- US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. FDAhttps://www.fda.gov/media/145802/download (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

