SASH3 variants cause a novel form of X-linked combined immunodeficiency with immune dysregulation
- PMID: 33876203
- PMCID: PMC8462359
- DOI: 10.1182/blood.2020008629
SASH3 variants cause a novel form of X-linked combined immunodeficiency with immune dysregulation
Abstract
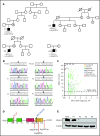
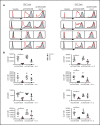
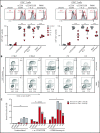
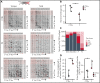
Sterile alpha motif (SAM) and Src homology-3 (SH3) domain-containing 3 (SASH3), also called SH3-containing lymphocyte protein (SLY1), is a putative adaptor protein that is postulated to play an important role in the organization of signaling complexes and propagation of signal transduction cascades in lymphocytes. The SASH3 gene is located on the X-chromosome. Here, we identified 3 novel SASH3 deleterious variants in 4 unrelated male patients with a history of combined immunodeficiency and immune dysregulation that manifested as recurrent sinopulmonary, cutaneous, and mucosal infections and refractory autoimmune cytopenias. Patients exhibited CD4+ T-cell lymphopenia, decreased T-cell proliferation, cell cycle progression, and increased T-cell apoptosis in response to mitogens. In vitro T-cell differentiation of CD34+ cells and molecular signatures of rearrangements at the T-cell receptor α (TRA) locus were indicative of impaired thymocyte survival. These patients also manifested neutropenia and B-cell and natural killer (NK)-cell lymphopenia. Lentivirus-mediated transfer of the SASH3 complementary DNA-corrected protein expression, in vitro proliferation, and signaling in SASH3-deficient Jurkat and patient-derived T cells. These findings define a new type of X-linked combined immunodeficiency in humans that recapitulates many of the abnormalities reported in mice with Sly1-/- and Sly1Δ/Δ mutations, highlighting an important role of SASH3 in human lymphocyte function and survival.
© 2021 by The American Society of Hematology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

