A novel and highly effective mitochondrial uncoupling drug in T-cell leukemia
- PMID: 33876224
- PMCID: PMC8525334
- DOI: 10.1182/blood.2020008955
A novel and highly effective mitochondrial uncoupling drug in T-cell leukemia
Abstract
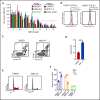
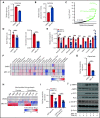
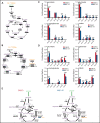
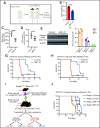
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy. Despite recent advances in treatments with intensified chemotherapy regimens, relapse rates and associated morbidities remain high. In this context, metabolic dependencies have emerged as a druggable opportunity for the treatment of leukemia. Here, we tested the antileukemic effects of MB1-47, a newly developed mitochondrial uncoupling compound. MB1-47 treatment in T-ALL cells robustly inhibited cell proliferation via both cytostatic and cytotoxic effects as a result of compromised mitochondrial energy and metabolite depletion, which severely impaired nucleotide biosynthesis. Mechanistically, acute treatment with MB1-47 in primary leukemias promoted adenosine monophosphate-activated serine/threonine protein kinase (AMPK) activation and downregulation of mammalian target of rapamycin (mTOR) signaling, stalling anabolic pathways that support leukemic cell survival. Indeed, MB1-47 treatment in mice harboring either murine NOTCH1-induced primary leukemias or human T-ALL patient-derived xenografts (PDXs) led to potent antileukemic effects with a significant extension in survival without overlapping toxicities. Overall, our findings demonstrate a critical role for mitochondrial oxidative phosphorylation in T-ALL and uncover MB1-47-driven mitochondrial uncoupling as a novel therapeutic strategy for the treatment of this disease.
© 2021 by The American Society of Hematology.
Figures




References
-
- Litzow MR, Ferrando AA.. How I treat T-cell acute lymphoblastic leukemia in adults. Blood. 2015;126(7):833-841. - PubMed
-
- Hefazi M, Litzow MR.. Recent advances in the biology and treatment of T-cell acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(4):265–274. - PubMed
-
- Hunger SP, Mullighan CG.. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. - PubMed
-
- Kozlowski P, Åström M, Ahlberg L, et al. ; Swedish Adult ALL Group . High relapse rate of T cell acute lymphoblastic leukemia in adults treated with hyper-CVAD chemotherapy in Sweden. Eur J Haematol. 2014;92(5):377-381. - PubMed
-
- Fielding AK, Richards SM, Chopra R, et al. ; Eastern Cooperative Oncology Group . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944-950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

