Tenascin C Promotes Glioma Cell Malignant Behavior and Inhibits Chemosensitivity to Paclitaxel via Activation of the PI3K/AKT Signaling Pathway
- PMID: 33876384
- PMCID: PMC8349315
- DOI: 10.1007/s12031-021-01832-8
Tenascin C Promotes Glioma Cell Malignant Behavior and Inhibits Chemosensitivity to Paclitaxel via Activation of the PI3K/AKT Signaling Pathway
Abstract
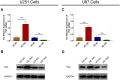
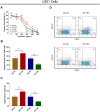
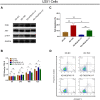
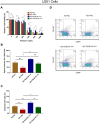
The present study aimed to detect the effect of tenascin C (TNC) on cell function and chemosensitivity to paclitaxel and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling in glioma cells.Human glioma cells U87, LN-229, T98G and U251 and normal human astrocytes were obtained, in which TNC expression was detected. The U87 cells and U251 cells were chosen and infected with lentivirus of control overexpression, TNC overexpression, control knockdown, and TNC knockdown for functional experiments. Rescue experiments were then performed to evaluate the effect of PI3K/AKT activator 740 Y-P on cell function and chemosensitivity to paclitaxel in TNC knockdown U251 cells. TNC mRNA and protein expression was elevated in glioma cells, including U87, LN-229, U251 and T98G cells, compared to normal human astrocytes. In U87 and U251 cells, TNC promoted proliferation while inhibiting apoptosis. In addition, TNC upregulated PI3K and p-AKT protein expression in U87 and U251 cells. As for chemosensitivity, TNC increased relative viability in U251 cells treated with 400 ng/mL and 800 ng/mL paclitaxel. In terms of stemness, TNC increased the sphere number per 1000 cells, CD44+CD133+ cell percentage and 1/stem cell frequency (assessed by extreme limiting dilution analysis) in U251 cells. In rescue experiments, 740 Y-P reduced the effect of TNC on proliferation, apoptosis, chemosensitivity to paclitaxel, and stemness in U251 cells. TNC acts as an oncogenic factor by promoting cancer cell proliferation and stemness while inhibiting apoptosis and chemosensitivity to paclitaxel in glioma via modulation of PI3K/AKT signaling.
Keywords: Cell function; Chemosensitivity; Glioma; PI3K/AKT signaling; Tenascin C.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures



References
-
- Bell EH, Zhang P, Shaw EG, Buckner JC, Barger GR, Bullard DE, Mehta MP, Gilbert MR, Brown PD, Stelzer KJ, McElroy JP, Fleming JL, Timmers CD, Becker AP, Salavaggione AL, Liu Z, Aldape K, Brachman DG, Gertler SZ, Murtha AD, Schultz CJ, Johnson D, Laack NN, Hunter GK, Crocker IR, Won M, Chakravarti A. Comprehensive Genomic Analysis in NRG Oncology/RTOG 9802: A Phase III Trial of Radiation Versus Radiation Plus Procarbazine, Lomustine (CCNU), and Vincristine in High-Risk Low-Grade Glioma. J Clin Oncol. 2020;38(29):3407–3417. doi: 10.1200/JCO.19.02983. - DOI - PMC - PubMed
-
- Buckner J, Pugh S, Shaw E, Gilbert M, Barger G, Coons S, Ricci P, Bullard D, Brown P, Stelzer K, Brachman D, Suh J, Schultz C, Bahary J-P, Fisher B, Kim H, Murtha A, Curran W, Mehta M. Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG. J Clin Oncol. 2014;32:2000–2000. doi: 10.1200/jco.2014.32.15_suppl.2000. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

