Take your Positions and Shine: Effects of Positioning Aggregation-Induced Emission Luminophores within Sequence-Defined Macromolecules
- PMID: 33876476
- PMCID: PMC8362002
- DOI: 10.1002/chem.202101086
Take your Positions and Shine: Effects of Positioning Aggregation-Induced Emission Luminophores within Sequence-Defined Macromolecules
Abstract
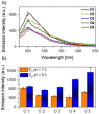
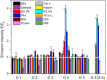
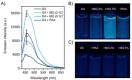
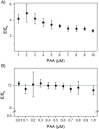
A luminophore with aggregation-induced emission (AIE) is employed for the conjugation onto supramolecular ligands to allow for detection of ligand binding. Supramolecular ligands are based on the combination of sequence-defined oligo(amidoamine) scaffolds and guanidiniocarbonyl-pyrrole (GCP) as binding motif. We hypothesize that AIE properties are strongly affected by positioning of the luminophore within the ligand scaffold. Therefore, we systematically investigate the effects placing the AIE luminophore at different positions within the overall construct, for example, in the main or side chain of the olig(amidoamine). Indeed, we can show that the position within the ligand structure strongly affects AIE, both for the ligand itself as well as when applying the ligand for the detection of different biological and synthetic polyanions.
Keywords: aggregation induced emission; biosensors; sequence-defined oligomers; solid phase polymer; supramolecular synthesis.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fang H., Xu S., Liu B., Adv. Mater. 2018, 30, 1801350. - PubMed
-
- Hong Y., Methods Appl. Fluoresc. 2016, 4, 022003. - PubMed
-
- Luo J., Xie Z., Lam J. W. Y., Cheng L., Chen H., Qiu C., Kwok H. S., Zhan X., Liu Y., Zhuc D., Tang B. Z., Chem. Commun. 2001, 18, 1740–1741. - PubMed
-
- Liang J., Tang B. Z., Liu B., Chem. Soc. Rev. 2015, 44, 2798–2811. - PubMed
-
- Zhao Z., Lam J. W. Y., Tang B. Z., J. Mater. Chem. 2012, 22, 23726–23740.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

