Intracellular amyloid hypothesis for ultra-early phase pathology of Alzheimer's disease
- PMID: 33876503
- PMCID: PMC8251586
- DOI: 10.1111/neup.12738
Intracellular amyloid hypothesis for ultra-early phase pathology of Alzheimer's disease
Abstract
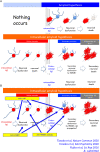
Using a new marker of necrosis, pSer46-MARCKS, which was identified by comprehensive phosphoproteome analysis as a phosphoprotein changed before appearance of extracellular amyloid aggregation, we discovered that neuronal necrosis occurs much earlier in Alzheimer's disease pathology than previously expected. The necrosis is induced by intracellular amyloid accumulation that deprives a critical effector molecule, Yes-associated protein (YAP), in the Hippo signaling pathway that is essential for cell survival, similarly to TRIAD necrosis observed in transcriptional repression and in other neurodegenerative diseases such as Huntington's disease. The initial TRIAD necrosis due to the intracellular amyloid releases HMGB1 into extracellular space and induces cluster of secondary necrosis around the primary necrotic neurons. Finally, the cluster grows into extracellular amyloid plaque. Inhibition of HMGB1 by anti-HMGB1 antibody prevents expansion of neurodegeneration. Administration even after onset significantly ameliorates the cognitive decline of Alzheimer's disease model mice. Our results present a new theory of Alzheimer's disease pathology, which can be referred to as the "intracellular amyloid hypothesis".
Keywords: Yes-associated protein (YAP); expansion of neurodegeneration; high mobility group box 1 (HMGB1); intracellular amyloid hypothesis; transcriptional repression-induced atypical cell death (TRIAD).
© 2021 The Author. Neuropathology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Neuropathology.
Figures


References
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci 1991; 12: 383–388. - PubMed
-
- Rinne JO, Brooks DJ, Rossor MN et al. 11C‐PiB PET assessment of change in fibrillar amyloid‐β load in patients with Alzheimer's disease treated with bapineuzumab: A phase 2, double‐blind, placebo‐controlled, ascending‐dose study. Lancet Neurol 2010; 9: 363–372. - PubMed
-
- Sevigny J, Chiao P, Bussière T et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature 2016; 537: 50–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

