The biphasic and age-dependent impact of klotho on hallmarks of aging and skeletal muscle function
- PMID: 33876724
- PMCID: PMC8118657
- DOI: 10.7554/eLife.61138
The biphasic and age-dependent impact of klotho on hallmarks of aging and skeletal muscle function
Abstract
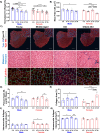
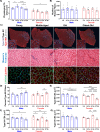
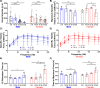
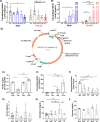
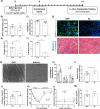
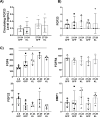
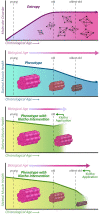
Aging is accompanied by disrupted information flow, resulting from accumulation of molecular mistakes. These mistakes ultimately give rise to debilitating disorders including skeletal muscle wasting, or sarcopenia. To derive a global metric of growing 'disorderliness' of aging muscle, we employed a statistical physics approach to estimate the state parameter, entropy, as a function of genes associated with hallmarks of aging. Escalating network entropy reached an inflection point at old age, while structural and functional alterations progressed into oldest-old age. To probe the potential for restoration of molecular 'order' and reversal of the sarcopenic phenotype, we systemically overexpressed the longevity protein, Klotho, via AAV. Klotho overexpression modulated genes representing all hallmarks of aging in old and oldest-old mice, but pathway enrichment revealed directions of changes were, for many genes, age-dependent. Functional improvements were also age-dependent. Klotho improved strength in old mice, but failed to induce benefits beyond the entropic tipping point.
Keywords: adeno-associated virus; computational biology; hallmarks of aging; klotho; mouse; muscle stem cells; regenerative medicine; sarcopenia; skeletal muscle; stem cells; systems biology.
© 2021, Clemens et al.
Conflict of interest statement
ZC, SS, AP, AS, SS, HM, NL, JC, PD, AB, FA No competing interests declared, JH, SK is an employee of Boehringer INgelheim Pharmaceutical Company, MF is an employee of Boehringer Ingelheim Pharmaceutical Company
Figures
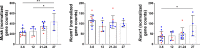

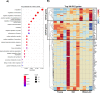
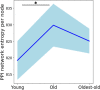
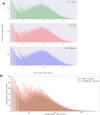
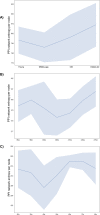
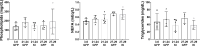





References
-
- Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Journal of Morphological Sciences. 2017;21:01
-
- Barns M, Gondro C, Tellam RL, Radley-Crabb HG, Grounds MD, Shavlakadze T. Molecular analyses provide insight into mechanisms underlying Sarcopenia and myofibre denervation in old skeletal muscles of mice. The International Journal of Biochemistry & Cell Biology. 2014;53:174–185. doi: 10.1016/j.biocel.2014.04.025. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

