Sensitivity of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population
- PMID: 33876726
- PMCID: PMC8081522
- DOI: 10.7554/eLife.65726
Sensitivity of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population
Abstract
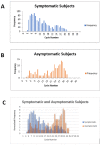
Diagnosis of SARS-CoV-2 (COVID-19) requires confirmation by reverse transcription-polymerase chain reaction (RT-PCR). Abbott ID NOW provides fast results but has been criticized for low sensitivity. Here we determine the sensitivity of ID NOW in an ambulatory population presented for testing. The study enrolled 785 symptomatic patients, of whom 21 were positive by both ID NOW and RT-PCR, and 2 only by RT-PCR. All 189 asymptomatic patients tested negative. The positive percent agreement between the ID NOW assay and the RT-PCR assay was 91.3%, and negative percent agreement was 100%. The results from the current study were included into a larger systematic review of literature where at least 20 subjects were simultaneously tested using ID NOW and RT-PCR. The overall sensitivity for ID NOW assay was calculated at 84% (95% confidence interval 55-96%) and had the highest correlation to RT-PCR at viral loads most likely to be associated with transmissible infections.
Keywords: COVID-19; ID NOW; RT-PCR; SARS-CoV-2; ambulatory; human; medicine; sensitivity.
Conflict of interest statement
YT, TO No competing interests declared, JI Reviewing editor, eLife
Figures

References
-
- Basu A, Zinger T, Inglima K, Woo KM, Atie O, Yurasits L, See B, Aguero-Rosenfeld ME. Performance of Abbott ID NOW COVID-19 rapid nucleic acid amplification test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York city academic institution. Journal of Clinical Microbiology. 2020;58:e01136-20. doi: 10.1128/JCM.01136-20. - DOI - PMC - PubMed
-
- Baughman AL, Bisgard KM, Cortese MM, Thompson WW, Sanden GN, Strebel PM. Utility of composite reference standards and latent class analysis in evaluating the clinical accuracy of diagnostic tests for pertussis. Clinical and Vaccine Immunology. 2008;15:106–114. doi: 10.1128/CVI.00223-07. - DOI - PMC - PubMed
-
- Comer SW, Fisk D. An extended laboratory validation study and comparative performance evaluation of the Abbott ID NOW COVID-19 assay in a coastal California tertiary care medical center. medRxiv. 2020 doi: 10.1101/2020.06.14.20130518. - DOI
-
- Cradic K, Lockhart M, Ozbolt P, Fatica L, Landon L, Lieber M, Yang D, Swickard J, Wongchaowart N, Fuhrman S, Antonara S. Clinical evaluation and utilization of multiple molecular in vitro diagnostic assays for the detection of SARS-CoV-2. American Journal of Clinical Pathology. 2020;207:aqaa097. doi: 10.1093/ajcp/aqaa097. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

