Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences
- PMID: 33876751
- PMCID: PMC8053943
- DOI: 10.1073/pnas.2016239118
Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences
Abstract
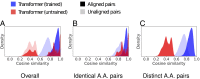
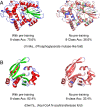
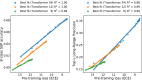
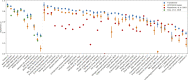
In the field of artificial intelligence, a combination of scale in data and model capacity enabled by unsupervised learning has led to major advances in representation learning and statistical generation. In the life sciences, the anticipated growth of sequencing promises unprecedented data on natural sequence diversity. Protein language modeling at the scale of evolution is a logical step toward predictive and generative artificial intelligence for biology. To this end, we use unsupervised learning to train a deep contextual language model on 86 billion amino acids across 250 million protein sequences spanning evolutionary diversity. The resulting model contains information about biological properties in its representations. The representations are learned from sequence data alone. The learned representation space has a multiscale organization reflecting structure from the level of biochemical properties of amino acids to remote homology of proteins. Information about secondary and tertiary structure is encoded in the representations and can be identified by linear projections. Representation learning produces features that generalize across a range of applications, enabling state-of-the-art supervised prediction of mutational effect and secondary structure and improving state-of-the-art features for long-range contact prediction.
Keywords: deep learning; generative biology; protein language model; representation learning; synthetic biology.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: A.R., J. Meier, S.G., D.G., M.O., C.L.Z., J. Ma, and R.F. are coinventors on a US patent application relating to the work of this manuscript.
Figures



References
-
- Yanofsky C., Horn V., Thorpe D., Protein structure relationships revealed by mutational analysis. Science 146, 1593–1594 (1964). - PubMed
-
- Altschuh D., Lesk A. M., Bloomer A. C., Klug A., Correlation of co-ordinated amino acid substitutions with function in viruses related to tobacco mosaic virus. J. Mol. Biol. 193, 693–707 (1987). - PubMed
-
- Altschuh D., Vernet T., Berti P., Moras D., Nagai K., Coordinated amino acid changes in homologous protein families. Protein Eng. 2, 193–199 (1988). - PubMed
-
- Göbel U., Sander C., Schneider R., Valencia A., Correlated mutations and residue contacts in proteins. Proteins 18, 309–317 (1994). - PubMed
-
- Harris Z. S., Distributional structure. Word 10, 146–162 (1954).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

