Modular complement assemblies for mitigating inflammatory conditions
- PMID: 33876753
- PMCID: PMC8054013
- DOI: 10.1073/pnas.2018627118
Modular complement assemblies for mitigating inflammatory conditions
Abstract
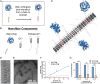
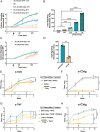
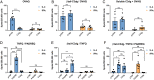
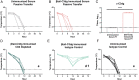
Complement protein C3dg, a key linkage between innate and adaptive immunity, is capable of stimulating both humoral and cell-mediated immune responses, leading to considerable interest in its use as a molecular adjuvant. However, the potential of C3dg as an adjuvant is limited without ways of controllably assembling multiple copies of it into vaccine platforms. Here, we report a strategy to assemble C3dg into supramolecular nanofibers with excellent compositional control, using β-tail fusion tags. These assemblies were investigated as therapeutic active immunotherapies, which may offer advantages over existing biologics, particularly toward chronic inflammatory diseases. Supramolecular assemblies based on the Q11 peptide system containing β-tail-tagged C3dg, B cell epitopes from TNF, and the universal T cell epitope PADRE raised strong antibody responses against both TNF and C3dg, and prophylactic immunization with these materials significantly improved protection in a lethal TNF-mediated inflammation model. Additionally, in a murine model of psoriasis induced by imiquimod, the C3dg-adjuvanted nanofiber vaccine performed as well as anti-TNF monoclonal antibodies. Nanofibers containing only β-tail-C3dg and lacking the TNF B cell epitope also showed improvements in both models, suggesting that supramolecular C3dg, by itself, played an important therapeutic role. We observed that immunization with β-tail-C3dg caused the expansion of an autoreactive C3dg-specific T cell population, which may act to dampen the immune response, preventing excessive inflammation. These findings indicate that molecular assemblies displaying C3dg warrant further development as active immunotherapies.
Keywords: active immunotherapy; immune engineering; immunoengineering; self-assembly; vaccine.
Conflict of interest statement
Competing interest statement: J.H.C. is an inventor on a US patent describing the β-tail technology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

