Posture-Dependent Collapse of the Optic Nerve Subarachnoid Space: A Combined MRI and Modeling Study
- PMID: 33877263
- PMCID: PMC8083083
- DOI: 10.1167/iovs.62.4.26
Posture-Dependent Collapse of the Optic Nerve Subarachnoid Space: A Combined MRI and Modeling Study
Abstract
Purpose: We hypothesize that a collapse of the optic nerve subarachnoid space (ONSAS) in the upright posture may protect the eyes from large translamina cribrosa pressure differences (TLCPD) believed to play a role in various optic nerve diseases (e.g., glaucoma). In this study, we combined magnetic resonance imaging (MRI) and mathematical modeling to investigate this potential ONSAS collapse and its effects on the TLCPD.
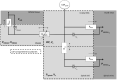
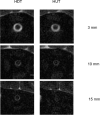
Methods: First, we performed MRI on six healthy volunteers in 6° head-down tilt (HDT) and 13° head-up tilt (HUT) to assess changes in ONSAS volume (measured from the eye to the optic canal) with changes in posture. The volume change reflects optic nerve sheath (ONS) distensibility. Second, we used the MRI data and mathematical modeling to simulate ONSAS pressure and the potential ONSAS collapse in a 90° upright posture.
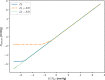
Results: The MRI showed a 33% decrease in ONSAS volume from the HDT to HUT (P < 0.001). In the upright posture, the simulations predicted an ONSAS collapse 25 mm behind lamina cribrosa, disrupting the pressure communication between the ONSAS and the intracranial subarachnoid space. The collapse reduced the simulated postural increase in TLCPD by roughly 1 mm Hg, although this reduction was highly sensitive to ONS distensibility, varying between 0 and 4.8 mm Hg when varying the distensibility by ± 1 SD.
Conclusions: The ONSAS volume along the optic nerve is posture dependent. The simulations supported the hypothesized ONSAS collapse in the upright posture and showed that even small changes in ONS stiffness/distensibility may affect the TLCPD.
Conflict of interest statement
Disclosure:
Figures





Comment in
-
Posture-Dependent Collapse of the Optic Nerve Subarachnoid Space: A Combined MRI and Modeling Study.Invest Ophthalmol Vis Sci. 2021 Dec 1;62(15):16. doi: 10.1167/iovs.62.15.16. Invest Ophthalmol Vis Sci. 2021. PMID: 34932064 Free PMC article. No abstract available.
-
Author Response: Posture-Dependent Collapse of the Optic Nerve Subarachnoid Space: A Combined MRI and Modeling Study.Invest Ophthalmol Vis Sci. 2021 Dec 1;62(15):15. doi: 10.1167/iovs.62.15.15. Invest Ophthalmol Vis Sci. 2021. PMID: 34932065 Free PMC article. No abstract available.
References
-
- Ren R, Jonas JB, Tian G, et al.. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010; 117: 259–266. - PubMed
-
- Lindén C, Qvarlander S, Jóhannesson G, et al.. Normal-tension glaucoma has normal intracranial pressure: a prospective study of intracranial pressure and intraocular pressure in different body positions. Ophthalmology. 2018; 125: 361–368. - PubMed
-
- Guy AH, Wiggs JL, Turalba A, Pasquale LR.. Translating the low translaminar cribrosa pressure gradient hypothesis into the clinical care of glaucoma. Semin Ophthalmol. 2016; 31: 131–139. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

