Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study
- PMID: 33877323
- PMCID: PMC8245892
- DOI: 10.1093/jn/nxab087
Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study
Abstract
Background: Curcuminoids from turmeric rhizome have significant health benefits but low bioavailability.
Objectives: To assess the pharmacokinetics of a novel natural turmeric dried colloidal suspension compared with 4 other turmeric formulations (including a standardized extract) at their respective recommended dosages.
Methods: Thirty healthy men and women (18 to 45 y old) were enrolled in a randomized, open-labeled, crossover trial, and sequentially consumed single oral doses of standard turmeric extract (1500 mg), liquid micellar preparation (1000 mg), piperine-curcuminoid combination (1515 mg), phytosome formulation (1000 mg), or the dried colloidal suspension (300 mg). Eleven blood samples were obtained over 24 h, plasma was extracted with or without deconjugation with β-glucuronidase or sulfatase, and ultra-high-pressure liquid chromatography/tandem MS was used to quantify the 3 parent curcuminoids and 12 metabolites. Classical pharmacokinetics parameters were derived.
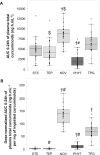
Results: The total AUC values of unconjugated curcuminoids were highly variable within participants, with no significant differences between formulations. However, the AUC values for total curcuminoids (including all metabolites) showed significant product effects. Indeed, the micellar preparation delivered higher levels of total curcuminoids than any other formulation (8540 ng·h/mL), reaching significance when compared with the dried colloidal suspension and standard extract (6520 and 5080 ng·h/mL, respectively). After dose normalization, both micellar and dried colloidal formulations showed significantly higher AUC levels than the standard extract (respectively 136 and 72.9, compared with 3.7 ng·h/mL/mg). Total curcuminoid absorption levels were also significantly higher for the dried colloidal suspension when compared with either piperine or phytosome formulations. Interestingly, no significant differences were observed between the piperine-curcuminoid combination and the standard extract. No serious adverse events were reported.
Conclusions: The administration of a low dose of the novel natural dried colloidal suspension provided high unconjugated and conjugated curcuminoid absorption, with significant beneficial differences when compared with the high dose of standard extract.This trial was registered at clinicaltrials.gov as NCT03621865.
Keywords: Curcuma longa; absorption; curcumin; curcuminoids; metabolism; metabolites; relative bioavailability.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures



Similar articles
-
Comparative bioavailability of curcuminoids from a water-dispersible high curcuminoid turmeric extract against a generic turmeric extract: a randomized, cross-over, comparative, pharmacokinetic study.J Pharm Pharmacol. 2021 Apr 27;73(6):816-823. doi: 10.1093/jpp/rgab028. J Pharm Pharmacol. 2021. PMID: 33755149 Clinical Trial.
-
Comparative Pharmacokinetics of Curcuminoids from Water-Dispersible Turmeric Extract Against a Curcuminoids-Piperine Combination: An Open-Label, Randomized, Balanced, 2-Treatment, 2-Sequence, 2-Period Crossover Study.Altern Ther Health Med. 2024 Apr;30(4):18-23. Altern Ther Health Med. 2024. PMID: 38702159 Clinical Trial.
-
Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers.Br J Clin Pharmacol. 2013 Feb;75(2):450-62. doi: 10.1111/j.1365-2125.2012.04364.x. Br J Clin Pharmacol. 2013. PMID: 22725836 Free PMC article. Clinical Trial.
-
Beyond Yellow Curry: Assessing Commercial Curcumin Absorption Technologies.J Am Coll Nutr. 2015;34(4):347-58. doi: 10.1080/07315724.2014.950392. Epub 2015 Apr 9. J Am Coll Nutr. 2015. PMID: 25856323 Free PMC article. Review.
-
Therapeutic potential of turmeric in Alzheimer's disease: curcumin or curcuminoids?Phytother Res. 2014 Apr;28(4):517-25. doi: 10.1002/ptr.5030. Epub 2013 Jul 19. Phytother Res. 2014. PMID: 23873854 Review.
Cited by
-
Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside.Int J Mol Sci. 2022 Mar 24;23(7):3557. doi: 10.3390/ijms23073557. Int J Mol Sci. 2022. PMID: 35408920 Free PMC article. Review.
-
The Therapeutic Effects of Bioactive Compounds on Colorectal Cancer via PI3K/Akt/mTOR Signaling Pathway: A Critical Review.Food Sci Nutr. 2024 Nov 7;12(12):9951-9973. doi: 10.1002/fsn3.4534. eCollection 2024 Dec. Food Sci Nutr. 2024. PMID: 39723045 Free PMC article. Review.
-
Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient's Loss of Consciousness.Int J Mol Sci. 2023 Apr 1;24(7):6621. doi: 10.3390/ijms24076621. Int J Mol Sci. 2023. PMID: 37047589 Free PMC article. Review.
-
Dry Heating of Curcumin in the Presence of Basic Salts Yields Anti-inflammatory Dimerization Products.ACS Omega. 2024 Aug 20;9(35):37025-37034. doi: 10.1021/acsomega.4c03257. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246485 Free PMC article.
-
Genistein and Procyanidin B2 Reduce Carcinogen-Induced Reactive Oxygen Species and DNA Damage through the Activation of Nrf2/ARE Cell Signaling in Bronchial Epithelial Cells In Vitro.Int J Mol Sci. 2023 Feb 12;24(4):3676. doi: 10.3390/ijms24043676. Int J Mol Sci. 2023. PMID: 36835090 Free PMC article.
References
-
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–7. - PubMed
-
- Smith T, Gillespie M, Eckl V, Knepper J, Morton Reynolds C. Herbal supplement sales in US increase by 9.4% in 2018. HerbalGram. 2019;123:62–73.
-
- Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87. - PubMed
-
- Bhattacharyya S, Mandal D, Sen GS, Pal S, Banerjee S, Lahiry L, Finke JH, Tannenbaum CS, Das T, Sa G. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. 2007;67:362–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

