Mimickers of breast malignancy: imaging findings, pathologic concordance and clinical management
- PMID: 33877461
- PMCID: PMC8058137
- DOI: 10.1186/s13244-021-00991-x
Mimickers of breast malignancy: imaging findings, pathologic concordance and clinical management
Abstract
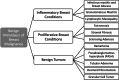
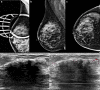
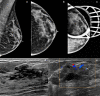
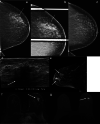
Many benign breast entities have a clinical and imaging presentation that can mimic breast cancer. The purpose of this review is to illustrate the wide spectrum of imaging features that can be associated with benign breast diseases with an emphasis on the suspicious imaging findings associated with these benign conditions that can mimic cancer. As radiologic-pathologic correlation can be particularly challenging in these cases, the radiologist's familiarity with these benign entities and their imaging features is essential to ensure that a benign pathology result is accepted as concordant when appropriate and that a suitable management plan is formulated.
Keywords: Benign breast disease; Benign breast masses; Breast cancer; Inflammatory breast disease; Radiologic-pathologic concordance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures











References
-
- Sabaté JM, Clotet M, Gómez A, De Las HP, Torrubia S, Salinas T. Radiologic evaluation of uncommon inflammatory and reactive breast disorders. Radiographics. 2005;25(2):411–424. - PubMed
-
- Trop I, Dugas A, David J, et al. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics. 2011;31(6):1683–1699. - PubMed
-
- Schäfer P, Fürrer C, Mermillod B. An association of cigarette smoking with recurrent subareolar breast abscess. Int J Epidemiol. 1988;17(4):810–813. - PubMed
-
- Rizzo M, Gabram S, Staley C, et al. Management of breast abscesses in nonlactating women. Am Surg. 2010;76(3):292–295. - PubMed
-
- Berna-Serna JD, Madrigal M. Percutaneous management of breast abscesses. An experience of 39 cases. Ultrasound Med Biol. 2004;30(1):1–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

