ROCK2 Inhibition With Belumosudil (KD025) for the Treatment of Chronic Graft-Versus-Host Disease
- PMID: 33877856
- PMCID: PMC8189612
- DOI: 10.1200/JCO.20.02754
ROCK2 Inhibition With Belumosudil (KD025) for the Treatment of Chronic Graft-Versus-Host Disease
Abstract
Purpose: The rho-associated coiled-coil-containing protein kinase-2 (ROCK2) signaling pathway regulates the Th17/regulatory T cells balance and controls profibrotic pathways. Selective ROCK2 inhibition with belumosudil (KD025) may offer a novel approach to the management of chronic graft-versus-host disease (cGVHD).
Patients and methods: A phase IIa, open-label, dose-finding study of belumosudil enrolled 54 patients with cGVHD who had received one to three prior lines of therapy (LOTs). The primary end point was overall response rate (ORR).
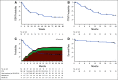
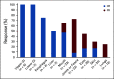
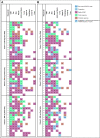
Results: The median time from cGVHD diagnosis to enrollment was 20 months. Seventy-eight percent of patients had severe cGVHD, 50% had ≥ 4 organs involved, 73% had cGVHD refractory to their last LOT, and 50% had received ≥ 3 prior LOTs. With an overall median follow-up of 29 months, the ORR (95% CI) with belumosudil 200 mg once daily, 200 mg twice daily, and 400 mg once daily was 65% (38% to 86%), 69% (41% to 89%), and 62% (38% to 82%), respectively. Responses were clinically meaningful, with a median duration of response of 35 weeks, and were associated with quality-of-life improvements and corticosteroid (CS) dose reductions. CS treatment was discontinued in 19% of patients. The failure-free survival rate was 76% (62% to 85%) and 47% (33% to 60%) at 6 and 12 months, respectively. The 2-year overall survival rate was 82% (69% to 90%). Belumosudil was well-tolerated, with low rates of cytopenia. There were no unexpected adverse events and no apparent increased risk of infection, including cytomegalovirus infection and reactivation.
Conclusion: Belumosudil treatment resulted in a high ORR and overall survival rate and demonstrated quality-of-life improvements, CS dose reductions, and limited toxicity. Data from the study indicated that belumosudil may prove to be an effective therapy for patients with treatment-refractory cGVHD.
Trial registration: ClinicalTrials.gov NCT02841995.
Conflict of interest statement
Figures





Comment in
-
Treating Inflammation and Fibrosis in Chronic GVHD: Two Birds, One ROCK.J Clin Oncol. 2021 Jun 10;39(17):1942-1945. doi: 10.1200/JCO.21.00214. Epub 2021 Apr 20. J Clin Oncol. 2021. PMID: 33877859 No abstract available.
-
Reply to A. Heine et al.J Clin Oncol. 2021 Oct 10;39(29):3308-3309. doi: 10.1200/JCO.21.01559. Epub 2021 Aug 4. J Clin Oncol. 2021. PMID: 34347529 No abstract available.
-
ROCKing Chronic Graft-Versus-Host Disease.J Clin Oncol. 2021 Oct 10;39(29):3308. doi: 10.1200/JCO.21.01081. Epub 2021 Aug 4. J Clin Oncol. 2021. PMID: 34347530 No abstract available.
Similar articles
-
Belumosudil for Chronic Graft-Versus-Host Disease: Analysis of Long-Term Results from the KD025-208 and ROCKstar Studies.Transplant Cell Ther. 2025 Jul;31(7):434.e1-434.e10. doi: 10.1016/j.jtct.2025.04.020. Epub 2025 May 1. Transplant Cell Ther. 2025. PMID: 40318736 Clinical Trial.
-
Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study.Blood. 2021 Dec 2;138(22):2278-2289. doi: 10.1182/blood.2021012021. Blood. 2021. PMID: 34265047 Free PMC article. Clinical Trial.
-
An open-label study of belumosudil, a selective ROCK2 inhibitor, as second or subsequent line of therapy for steroid-dependent/steroid-resistant chronic GVHD.Am J Hematol. 2024 Oct;99(10):1917-1926. doi: 10.1002/ajh.27424. Epub 2024 Jun 27. Am J Hematol. 2024. PMID: 38934629 Clinical Trial.
-
Belumosudil for chronic graft-versus-host disease.Drugs Today (Barc). 2022 May;58(5):203-212. doi: 10.1358/dot.2022.58.5.3400705. Drugs Today (Barc). 2022. PMID: 35535812 Review.
-
Belumosudil: First Approval.Drugs. 2021 Sep;81(14):1677-1682. doi: 10.1007/s40265-021-01593-z. Drugs. 2021. PMID: 34463931 Free PMC article. Review.
Cited by
-
Recent advances in graft-versus-host disease.Fac Rev. 2023 Mar 6;12:4. doi: 10.12703/r/12-4. eCollection 2023. Fac Rev. 2023. PMID: 36923700 Free PMC article. Review.
-
Recent FDA Approvals in the Treatment of Graft-Versus-Host Disease.Oncologist. 2022 Aug 5;27(8):685-693. doi: 10.1093/oncolo/oyac076. Oncologist. 2022. PMID: 35443042 Free PMC article. Review.
-
Analyses and Correlation of Pathologic and Ocular Cutaneous Changes in Murine Graft versus Host Disease.Int J Mol Sci. 2021 Dec 24;23(1):184. doi: 10.3390/ijms23010184. Int J Mol Sci. 2021. PMID: 35008621 Free PMC article.
-
Safety and efficacy of the ROCK-2-inhibitor Belumosudil in cGvHD treatment - a retrospective, German-Swiss multicenter real-world data analysis.Bone Marrow Transplant. 2025 Apr;60(4):439-446. doi: 10.1038/s41409-024-02507-9. Epub 2025 Jan 14. Bone Marrow Transplant. 2025. PMID: 39809902 Free PMC article.
-
Attenuation of apoptotic cell detection triggers thymic regeneration after damage.Cell Rep. 2021 Oct 5;37(1):109789. doi: 10.1016/j.celrep.2021.109789. Cell Rep. 2021. PMID: 34610317 Free PMC article.
References
-
- Grube M Holler E Weber D, et al. : Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation—Results from a single-center observational study. Biol Blood Marrow Transpl 22:1781-1791, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

