Brain network mechanisms of visual shape completion
- PMID: 33878383
- PMCID: PMC8456451
- DOI: 10.1016/j.neuroimage.2021.118069
Brain network mechanisms of visual shape completion
Abstract
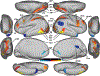
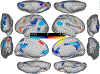
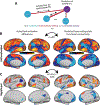
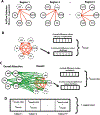
Visual shape completion recovers object shape, size, and number from spatially segregated edges. Despite being extensively investigated, the process's underlying brain regions, networks, and functional connections are still not well understood. To shed light on the topic, we scanned (fMRI) healthy adults during rest and during a task in which they discriminated pac-man configurations that formed or failed to form completed shapes (illusory and fragmented condition, respectively). Task activation differences (illusory-fragmented), resting-state functional connectivity, and multivariate patterns were identified on the cortical surface using 360 predefined parcels and 12 functional networks composed of such parcels. Brain activity flow mapping (ActFlow) was used to evaluate the likely involvement of resting-state connections for shape completion. We identified 36 differentially-active parcels including a posterior temporal region, PH, whose activity was consistent across 95% of observers. Significant task regions primarily occupied the secondary visual network but also incorporated the frontoparietal, dorsal attention, default mode, and cingulo-opercular networks. Each parcel's task activation difference could be modeled via its resting-state connections with the remaining parcels (r=.62, p<10-9), suggesting that such connections undergird shape completion. Functional connections from the dorsal attention network were key in modelling task activation differences in the secondary visual network. Dorsal attention and frontoparietal connections could also model activations in the remaining networks. Taken together, these results suggest that shape completion relies upon a sparsely distributed but densely interconnected network coalition that is centered in the secondary visual network, coordinated by the dorsal attention network, and inclusive of at least three other networks.
Keywords: Area PH; Dorsal attention network; Frontoparietal network; Kanizsa shapes; Resting-state functional connectivity; Secondary visual network; Subjective contours.
Copyright © 2021. Published by Elsevier Inc.
Figures



References
-
- Beck J, 1966. Effect of orientation and of shape similarity on perceptual grouping. Percept. Psychophys 1, 300–302.
-
- Benjamini Y, Hochberg J, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B-Methodol 57, 289–300.
-
- Bhandari R, Kirilina E, Caan M, Suttrup J, De Sanctis T, De Angelis L, Keysers C, Gazzola V, 2020. Does higher sampling rate (multiband + SENSE) improve group statistics - an example from social neuroscience block design at 3T. Neuroimage 213, 116731. doi: 10.1016/j.neuroimage.2020.116731. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

