Identification of prognostic biomarkers associated with the occurrence of portal vein tumor thrombus in hepatocellular carcinoma
- PMID: 33878734
- PMCID: PMC8109071
- DOI: 10.18632/aging.202876
Identification of prognostic biomarkers associated with the occurrence of portal vein tumor thrombus in hepatocellular carcinoma
Abstract
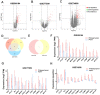
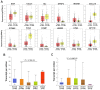
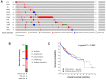
The occurrence of portal vein tumor thrombus (PVTT) is strongly correlated to the staging and poor prognosis of hepatocellular carcinoma (HCC) patients. However, the mechanisms of PVTT formation remain unclear. This study aimed to investigate differentially expressed genes (DEGs) between primary tumor (PT) and PVTT tissues and comprehensively explored the underlying mechanisms of PVTT formation. The DEGs between PT and paired PVTT tissues were analyzed using transcriptional data from the Gene Expression Omnibus (GEO) database. The expression, clinical relevance, prognostic significance, genetic alternations, DNA methylation, correlations with immune infiltration, co-expression correlations, and functional enrichment analysis of the DEGs were explored using multiple databases. As result, 12 DEGs were commonly down-expressed in PVTT compared with PT tissues among three datasets. The expression of DCN, CCL21, IGJ, CXCL14, FCN3, LAMA2, and NPY1R was progressively decreased from normal liver, PT, to PVTT tissues, whose up-expression associated with favorable survivals of HCC patients. The genetic alternations and DNA methylation of the DEGs frequently occurred, and several methylated CpG sites of the DEGs significantly correlated with outcomes of HCC patients. The immune infiltration in the tumor microenvironment of HCC was correlated with the expression level of the DEGs. Besides, the DEGs and their co-expressive genes participated in the biological processes of extracellular matrix (ECM) organization and focal adhesion. In summary, this study indicated the dysregulation of ECM and focal adhesion might contribute to the formation of PVTT. And the above seven genes might serve as potential biomarkers of PVTT occurrence and prognosis of HCC patients.
Keywords: bioinformatics; hepatocellular carcinoma; immune infiltration; portal vein tumor thrombus; prognosis.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

