Bidirectional interconversion between PtdIns4P and PtdIns(4,5)P2 is required for autophagic lysosome reformation and protection from skeletal muscle disease
- PMID: 33879025
- PMCID: PMC8143231
- DOI: 10.1080/15548627.2021.1916195
Bidirectional interconversion between PtdIns4P and PtdIns(4,5)P2 is required for autophagic lysosome reformation and protection from skeletal muscle disease
Abstract
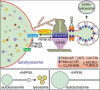
Autophagic lysosome reformation (ALR) recycles autolysosome membranes formed during autophagy, to make lysosomes and is essential for continued autophagy function. Localized membrane remodeling on autolysosomes leads to the extension of reformation tubules, which undergo scission to form new lysosomes. The phosphoinositides phosphatidylinositol-4-phosphate (PtdIns4P) and phosphatidylinositol-4,5-bisphosphate (PtdIns[4,5]P2) induce this remodeling by recruiting protein effectors to membranes. We identified the inositol polyphosphate 5-phosphatase INPP5K, which converts PtdIns(4,5)P2 to PtdIns4P is essential for ALR in skeletal muscle. INPP5K mutations that reduce its 5-phosphatase activity are known to cause muscular dystrophy, via an undefined mechanism. We generated skeletal muscle-specific inpp5k knockout mice which exhibited severe muscle disease, with lysosome depletion and marked autophagy inhibition. This was due to decreased PtdIns4P and increased PtdIns(4,5)P2 on autolysosomes, causing reduced scission of reformation tubules. ALR was restored in cells with loss of INPP5K by expression of wild-type INPP5K, but not muscle-disease causing mutants. Therefore on autolysosomes, both PtdIns(4,5)P2 generation and its removal by INPP5K is required for completion of ALR. Furthermore, skeletal muscle shows a dependence on the membrane recycling ALR pathway to maintain lysosome homeostasis and ensure the protective role of autophagy against disease.
Keywords: Autophagic lysosome reformation; INPP5K; PtdIns(4, 5)P2; PtdIns4P; autophagy; inositol polyphosphate 5-phosphatase; lysosome; muscular dystrophy; phosphoinositide; skeletal muscle.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Comment on
-
Defective lysosome reformation during autophagy causes skeletal muscle disease.J Clin Invest. 2021 Jan 4;131(1):e135124. doi: 10.1172/JCI135124. J Clin Invest. 2021. PMID: 33119550 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
