RB1 and TP53 co-mutations correlate strongly with genomic biomarkers of response to immunity checkpoint inhibitors in urothelial bladder cancer
- PMID: 33879103
- PMCID: PMC8056512
- DOI: 10.1186/s12885-021-08078-y
RB1 and TP53 co-mutations correlate strongly with genomic biomarkers of response to immunity checkpoint inhibitors in urothelial bladder cancer
Abstract
Background: Muscle invasive urothelial bladder carcinoma (MIBC) present RB1 and TP53 somatic alterations in a variable percentage of tumors throughout all molecular subtypes. MIBCs with neuroendocrine features have a high response rate to immunity checkpoint inhibitors (ICIs). Whether the presence of somatic co-alterations in these 2 genes in MIBCs is relevant to their responsiveness to ICIs is not known.
Methods: The potential correlation of different genomic biomarkers of response to ICIs like tumor mutational burden (TMB), single nucleotide variants (SNV) predicted neoantigens, DNA damage response (DDR) genes, DNA somatic signatures and TILs infiltrate was explored in patients with somatic co-alterations in RB1 and TP53 (RB1&TP53) as compared with patients with no alterations in any (double wild type, DWT) or with alterations in just one of the 2 genes. The Cancer Genome Atlas (TCGA) pancancer BLCA dataset of cystectomy specimens (n = 407) with mutation, copy number alterations and transcriptomic (RNA sequencing) data as well as the IMVigor 210 study (n = 348) of metastatic urothelial bladder cancers treated with atezolizumab (PD-L1 inhibitor) with clinical response data containing transcriptomic (RNA sequencing), along with a subset (n = 274) with mutation and copy number data were used for this purpose. A novel tumor microenvironment metascore (TMM) was developed based in a LASSO regularized Cox model with predictive and prognostic ability.
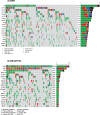
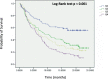
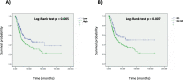
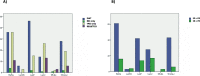
Results: Samples with co-altered RB1&TP53: a) were enriched in immunity effectors (CD8 cytotoxic lymphocytes, NK cells) and display higher scores of a T cell inflamed signature; b) have a higher TMB, higher number of SNV predicted neoantigens and higher TILs fractions; c) have a higher number of DDR mutated and deep deleted DDR genes; d) have DNA somatic signatures 2 and 13 related to APOBEC mutagenesis. Using the IMVigor 210 dataset, RB1&TP53 samples had the highest response rate to atezolizumab and a strong correlation with TMB and TMM. The consensus molecular subtype classification in the IMVigor 210 dataset showed a significant correlation with both the response to treatment (p = 0.001, Chisquare) and the presence of RB1 and TP53 genomic alterations (p < 0.001, Chisquare).
Conclusions: RB1&TP53 co-alterations are strongly associated with genomic biomarkers of response to ICIs in MIBCs.
Keywords: Bladder; Cancer; Co-mutation; Immunity checkpoint inhibitor; RB1; Signature; TP53; Urothelial.
Conflict of interest statement
The authors declare that they have no competing interests
Figures



Similar articles
-
Identification of a novel immune microenvironment signature predicting survival and therapeutic options for bladder cancer.Aging (Albany NY). 2020 Dec 19;13(2):2780-2802. doi: 10.18632/aging.202327. Epub 2020 Dec 19. Aging (Albany NY). 2020. PMID: 33408272 Free PMC article.
-
Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition.Cancer Control. 2020 Jan-Dec;27(1):1073274820976665. doi: 10.1177/1073274820976665. Cancer Control. 2020. PMID: 33356494 Free PMC article.
-
Specific TP53 subtype as biomarker for immune checkpoint inhibitors in lung adenocarcinoma.EBioMedicine. 2020 Oct;60:102990. doi: 10.1016/j.ebiom.2020.102990. Epub 2020 Sep 11. EBioMedicine. 2020. PMID: 32927274 Free PMC article.
-
Genetic Alterations in the Molecular Subtypes of Bladder Cancer: Illustration in the Cancer Genome Atlas Dataset.Eur Urol. 2017 Sep;72(3):354-365. doi: 10.1016/j.eururo.2017.03.010. Epub 2017 Mar 30. Eur Urol. 2017. PMID: 28365159 Free PMC article. Review.
-
The evolution of bladder cancer genomics: What have we learned and how can we use it?Urol Oncol. 2018 Jul;36(7):313-320. doi: 10.1016/j.urolonc.2018.02.017. Epub 2018 Mar 21. Urol Oncol. 2018. PMID: 29573965 Review.
Cited by
-
Identifying tumor immunity-associated molecular features in liver hepatocellular carcinoma by multi-omics analysis.Front Mol Biosci. 2022 Oct 20;9:960457. doi: 10.3389/fmolb.2022.960457. eCollection 2022. Front Mol Biosci. 2022. PMID: 36339710 Free PMC article.
-
Concurrent RB1 Loss and BRCA Deficiency Predicts Enhanced Immunologic Response and Long-term Survival in Tubo-ovarian High-grade Serous Carcinoma.Clin Cancer Res. 2024 Aug 15;30(16):3481-3498. doi: 10.1158/1078-0432.CCR-23-3552. Clin Cancer Res. 2024. PMID: 38837893 Free PMC article.
-
Integrated bioinformatics analysis identifies a Ferroptosis-related gene signature as prognosis model and potential therapeutic target of bladder cancer.Toxicol Res (Camb). 2024 Jan 27;13(1):tfae010. doi: 10.1093/toxres/tfae010. eCollection 2024 Feb. Toxicol Res (Camb). 2024. PMID: 38292893 Free PMC article.
-
Concurrent RB1 loss and BRCA-deficiency predicts enhanced immunological response and long-term survival in tubo-ovarian high-grade serous carcinoma.medRxiv [Preprint]. 2023 Nov 10:2023.11.09.23298321. doi: 10.1101/2023.11.09.23298321. medRxiv. 2023. Update in: Clin Cancer Res. 2024 Aug 15;30(16):3481-3498. doi: 10.1158/1078-0432.CCR-23-3552. PMID: 37986741 Free PMC article. Updated. Preprint.
-
Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review.Cancers (Basel). 2024 Sep 1;16(17):3056. doi: 10.3390/cancers16173056. Cancers (Basel). 2024. PMID: 39272913 Free PMC article. Review.
References
-
- Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol. 2018:Jco1801148. - PubMed
-
- Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (London, England) 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. - DOI - PubMed
-
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. - DOI - PMC - PubMed
-
- Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Szabados B, Pous AF, Gravis G, Herranz UA, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706–1714. doi: 10.1038/s41591-019-0628-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

