Autophagy blockade synergistically enhances nanosonosensitizer-enabled sonodynamic cancer nanotherapeutics
- PMID: 33879173
- PMCID: PMC8056542
- DOI: 10.1186/s12951-021-00855-y
Autophagy blockade synergistically enhances nanosonosensitizer-enabled sonodynamic cancer nanotherapeutics
Abstract
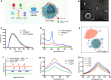
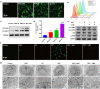
Ultrasound-triggered sonodynamic therapy (SDT) represents an emerging therapeutic modality for cancer treatment based on its specific feature of noninvasiveness, high tissue-penetrating depth and desirable therapeutic efficacy, but the SDT-induced pro-survival cancer-cell autophagy would significantly lower the SDT efficacy for cancer treatment. Here we propose an "all-in-one" combined tumor-therapeutic strategy by integrating nanosonosensitizers-augmented noninvasive SDT with autophagy inhibition based on the rationally constructed nanoliposomes that co-encapsulates clinically approved sonosensitizers protoporphyrin IX (PpIX) and early-phase autophagy-blocking agent 3-methyladenine (3-MA). It has been systematically demonstrated that nanosonosensitizers-augmented SDT induced cytoprotective pro-survival autophagy through activation of MAPK signaling pathway and inhibition of AMPK signaling pathway, and this could be efficaciously inhibited by 3-MA in early-phase autophagy, which significantly decreased the cell resistance to intracellular oxidative stress and complied a remarkable synergistic effect on SDT medicated cancer-cell apoptosis both in vitro at cellular level and in vivo on tumor-bearing animal model. Therefore, our results provide a proof-of-concept combinatorial tumor therapeutics based on nanosonosensitizers for the treatment of ROS-resistant cancer by autophagy inhibition-augmented SDT.
Keywords: Autophagy; Autophagy inhibition; Nanoliposomes; Sonodynamic therapy; Tumor therapy.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

