Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease
- PMID: 33879495
- PMCID: PMC8546968
- DOI: 10.1128/mSystems.00111-21
Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease
Abstract
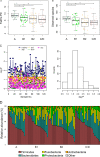
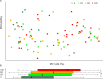
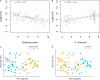
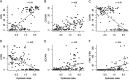
Gut dysbiosis and gut microbiota-derived metabolites, including bile acid (BA), short-chain fatty acid, and trimethylamine N-oxide (TMAO), are associated with cardiovascular disease. Canine myxomatous mitral valve disease (MMVD) is a model for human MMVD. The aim of the study is to evaluate gut microbial dysbiosis and its relationship with gut-produced metabolites in dogs with MMVD. Fecal samples from 92 privately owned dogs, including 17 healthy, 23 and 27 asymptomatic MMVD dogs without (stage B1) and with (stage B2) secondary cardiac enlargement, respectively, and 25 MMVD dogs with history of congestive heart failure (stage C or D), were analyzed by 16S rRNA sequencing. Alpha and beta diversities were different between healthy and MMVD dogs (adjusted P < 0.05). The average dysbiosis indexes were -1.48, -0.6, 0.01, and 1.47 for healthy, B1, B2, and C/D dogs, respectively (P = 0.07). Dysbiosis index was negatively correlated with Clostridium hiranonis (P < 0.0001, r = -0.79). Escherichia coli, capable of trimethylamine production in the gut, had an increased abundance (adjusted P < 0.05) and may be responsible for the increased circulating TMAO levels in stage B2 and C/D MMVD dogs. Primary and secondary BAs showed opposite associations with C. hiranonis, a key BA converter (P < 0.0001 for both, r = -0.94 and 0.95, respectively). Secondary BAs appeared to promote the growth of Fusobacterium and Faecalibacterium but inhibit that of E. coli Multivariate analysis revealed significant but weak associations between gut microbiota and several circulating metabolites, including short-chain acylcarnitines and TMAO.IMPORTANCE Our study expands the current "gut hypothesis" to include gut dysbiosis at the preclinical stage, prior to the onset of heart failure. Gut dysbiosis index increases in proportion to the severity of myxomatous mitral valve disease (MMVD) and is inversely associated with Clostridium hiranonis, a key bile acid (BA) converter in the gut. Secondary BAs appear to promote the growth of beneficial bacteria but inhibit that of harmful ones. An intricate interplay between gut microbiota, gut microbiota-produced metabolites, and MMVD pathophysiological progression is implicated.
Keywords: Clostridium hiranonis; bile acid; canine; congestive heart failure; dysbiosis; metabolite; microbial metabolite; microbiome; microbiota; mitral valve disease; trimethylamine N-oxide.
Copyright © 2021 Li et al.
Figures


Similar articles
-
Metabolomics Analysis Reveals Deranged Energy Metabolism and Amino Acid Metabolic Reprogramming in Dogs With Myxomatous Mitral Valve Disease.J Am Heart Assoc. 2021 May 4;10(9):e018923. doi: 10.1161/JAHA.120.018923. Epub 2021 Apr 23. J Am Heart Assoc. 2021. PMID: 33890477 Free PMC article.
-
Comparison of Intestinal Microbiota Between Healthy and MMVD Chihuahuas Using 16S rRNA Gene Amplicon Sequencing.Front Vet Sci. 2022 Mar 30;9:846492. doi: 10.3389/fvets.2022.846492. eCollection 2022. Front Vet Sci. 2022. PMID: 35433906 Free PMC article.
-
Gut Microbiota Dysbiosis Is Associated with Altered Bile Acid Metabolism in Infantile Cholestasis.mSystems. 2019 Dec 17;4(6):e00463-19. doi: 10.1128/mSystems.00463-19. mSystems. 2019. PMID: 31848302 Free PMC article.
-
A scoping review evaluating the current state of gut microbiota and its metabolites in valvular heart disease physiopathology.Eur J Clin Invest. 2025 Jun;55(6):e14381. doi: 10.1111/eci.14381. Epub 2025 Jan 10. Eur J Clin Invest. 2025. PMID: 39797472
-
Genetics of canine myxomatous mitral valve disease.Anim Genet. 2021 Aug;52(4):409-421. doi: 10.1111/age.13082. Epub 2021 May 24. Anim Genet. 2021. PMID: 34028063 Review.
Cited by
-
Effects of Postbiotic Administration on Canine Health: A Systematic Review and Meta-Analysis.Microorganisms. 2025 Jul 3;13(7):1572. doi: 10.3390/microorganisms13071572. Microorganisms. 2025. PMID: 40732081 Free PMC article. Review.
-
Collaborative Metabolism: Gut Microbes Play a Key Role in Canine and Feline Bile Acid Metabolism.Vet Sci. 2024 Feb 18;11(2):94. doi: 10.3390/vetsci11020094. Vet Sci. 2024. PMID: 38393112 Free PMC article. Review.
-
Effects of dietary macronutrient profile on apparent total tract macronutrient digestibility and fecal microbiota, fermentative metabolites, and bile acids of female dogs after spay surgery.J Anim Sci. 2021 Sep 1;99(9):skab225. doi: 10.1093/jas/skab225. J Anim Sci. 2021. PMID: 34333604 Free PMC article.
-
Unravelling the Gut Microbiome Role in Cardiovascular Disease: A Systematic Review and a Meta-Analysis.Biomolecules. 2024 Jun 20;14(6):731. doi: 10.3390/biom14060731. Biomolecules. 2024. PMID: 38927134 Free PMC article.
-
Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs.J Anim Sci. 2022 Feb 1;100(2):skab379. doi: 10.1093/jas/skab379. J Anim Sci. 2022. PMID: 34967874 Free PMC article.
References
-
- Buchanan J. 1999. Prevalence of cardiovascular disorders, p 457–470. In Sisson D, Fox PR, Sydney M (ed), Textbook of canine and feline cardiology, 2nd ed. Saunders, Philadelphia, PA.
LinkOut - more resources
Full Text Sources
Other Literature Sources

