Predicting disability progression and cognitive worsening in multiple sclerosis using patterns of grey matter volumes
- PMID: 33879535
- PMCID: PMC8372398
- DOI: 10.1136/jnnp-2020-325610
Predicting disability progression and cognitive worsening in multiple sclerosis using patterns of grey matter volumes
Abstract
Objective: In multiple sclerosis (MS), MRI measures at the whole brain or regional level are only modestly associated with disability, while network-based measures are emerging as promising prognostic markers. We sought to demonstrate whether data-driven patterns of covarying regional grey matter (GM) volumes predict future disability in secondary progressive MS (SPMS).
Methods: We used cross-sectional structural MRI, and baseline and longitudinal data of Expanded Disability Status Scale, Nine-Hole Peg Test (9HPT) and Symbol Digit Modalities Test (SDMT), from a clinical trial in 988 people with SPMS. We processed T1-weighted scans to obtain GM probability maps and applied spatial independent component analysis (ICA). We repeated ICA on 400 healthy controls. We used survival models to determine whether baseline patterns of covarying GM volume measures predict cognitive and motor worsening.
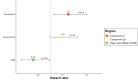
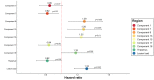
Results: We identified 15 patterns of regionally covarying GM features. Compared with whole brain GM, deep GM and lesion volumes, some ICA components correlated more closely with clinical outcomes. A mainly basal ganglia component had the highest correlations at baseline with the SDMT and was associated with cognitive worsening (HR=1.29, 95% CI 1.09 to 1.52, p<0.005). Two ICA components were associated with 9HPT worsening (HR=1.30, 95% CI 1.06 to 1.60, p<0.01 and HR=1.21, 95% CI 1.01 to 1.45, p<0.05). ICA measures could better predict SDMT and 9HPT worsening (C-index=0.69-0.71) compared with models including only whole and regional MRI measures (C-index=0.65-0.69, p value for all comparison <0.05).
Conclusions: The disability progression was better predicted by some of the covarying GM regions patterns, than by single regional or whole-brain measures. ICA, which may represent structural brain networks, can be applied to clinical trials and may play a role in stratifying participants who have the most potential to show a treatment effect.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
