Natalizumab, Fingolimod and Dimethyl Fumarate Use and Pregnancy-Related Relapse and Disability in Women With Multiple Sclerosis
- PMID: 33879599
- PMCID: PMC8253565
- DOI: 10.1212/WNL.0000000000012084
Natalizumab, Fingolimod and Dimethyl Fumarate Use and Pregnancy-Related Relapse and Disability in Women With Multiple Sclerosis
Abstract
Objective: To investigate pregnancy-related disease activity in a contemporary multiple sclerosis (MS) cohort.
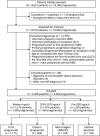
Methods: Using data from the MSBase Registry, we included pregnancies conceived after 31 Dec 2010 from women with relapsing-remitting MS or clinically isolated syndrome. Predictors of intrapartum relapse, and postpartum relapse and disability progression were determined by clustered logistic regression or Cox regression analyses.
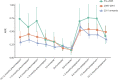
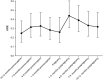
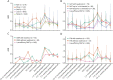
Results: We included 1998 pregnancies from 1619 women with MS. Preconception annualized relapse rate (ARR) was 0.29 (95% CI 0.27-0.32), fell to 0.19 (0.14-0.24) in third trimester, and increased to 0.59 (0.51-0.67) in early postpartum. Among women who used fingolimod or natalizumab, ARR before pregnancy was 0.37 (0.28-0.49) and 0.29 (0.22-0.37), respectively, and increased during pregnancy. Intrapartum ARR decreased with preconception dimethyl fumarate use. ARR spiked after delivery across all DMT groups. Natalizumab continuation into pregnancy reduced the odds of relapse during pregnancy (OR 0.76 per month [0.60-0.95], p=0.017). DMT re-initiation with natalizumab protected against postpartum relapse (HR 0.11 [0.04-0.32], p<0.0001). Breastfeeding women were less likely to relapse (HR 0.61 [0.41-0.91], p=0.016). 5.6% of pregnancies were followed by confirmed disability progression, predicted by higher relapse activity in pregnancy and postpartum.
Conclusion: Intrapartum and postpartum relapse probabilities increased among women with MS after natalizumab or fingolimod cessation. In women considered to be at high relapse risk, use of natalizumab before pregnancy and continued up to 34 weeks gestation, with early re-initiation after delivery is an effective option to minimize relapse risks. Strategies of DMT use have to be balanced against potential fetal/neonatal complications.
© 2021 American Academy of Neurology.
Figures
References
-
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis: Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285-291. - PubMed
-
- Nguyen AL, Eastaugh A, van der Walt A, Jokubaitis VG. Pregnancy and multiple sclerosis: clinical effects across the lifespan. Autoimmun Rev. 2019;18:102360. - PubMed
-
- Karp I, Manganas A, Sylvestre M-P, Ho A, Roger E, Duquette P. Does pregnancy alter the long-term course of multiple sclerosis? Ann Epidemiol. 2014;24:504-508.e2. - PubMed
-
- De Angelis F, John NA, Brownlee WJ. Disease-modifying therapies for multiple sclerosis. BMJ. 2018;363:k4674. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
