Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma
- PMID: 33879601
- PMCID: PMC8061836
- DOI: 10.1136/jitc-2021-002478
Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma
Abstract
Background: Merkel cell carcinoma (MCC) is an aggressive skin cancer associated with poor survival. Programmed cell death-1 (PD-1) pathway inhibitors have shown high rates of durable tumor regression compared with chemotherapy for MCC. The current study was undertaken to assess baseline and on-treatment factors associated with MCC regression and 3-year survival, and to explore the effects of salvage therapies in patients experiencing initial non-response or tumor progression after response or stable disease following first-line pembrolizumab therapy on Cancer Immunotherapy Trials Network-09/KEYNOTE-017.
Methods: In this multicenter phase II trial, 50 patients with advanced unresectable MCC received pembrolizumab 2 mg/kg every 3 weeks for ≤2 years. Patients were followed for a median of 31.8 months.
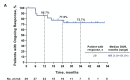
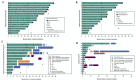
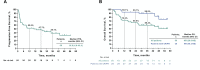
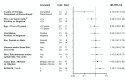
Results: Overall response rate to pembrolizumab was 58% (complete response 30%+partial response 28%; 95% CI 43.2 to 71.8). Among 29 responders, the median response duration was not reached (NR) at 3 years (range 1.0+ to 51.8+ months). Median progression-free survival (PFS) was 16.8 months (95% CI 4.6 to 43.4) and the 3-year PFS was 39.1%. Median OS was NR; the 3-year OS was 59.4% for all patients and 89.5% for responders. Baseline Eastern Cooperative Oncology Group performance status of 0, greater per cent tumor reduction, completion of 2 years of treatment and low neutrophil-to-lymphocyte ratio were associated with response and longer survival. Among patients with initial disease progression or those who developed progression after response or stable disease, some had extended survival with subsequent treatments including chemotherapies and immunotherapies.
Conclusions: This study represents the longest available follow-up from any first-line anti-programmed death-(ligand) 1 (anti-PD-(L)1) therapy in MCC, confirming durable PFS and OS in a proportion of patients. After initial tumor progression or relapse following response, some patients receiving salvage therapies survived. Improving the management of anti-PD-(L)1-refractory MCC remains a challenge and a high priority.
Trial registration number: NCT02267603.
Keywords: immunotherapy; programmed cell death 1 receptor; skin neoplasms.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PN reports grants from Bristol-Myers Squibb and EMD-Serono; advisory fees from EMD-Serono, Pfizer and Merck & Co.; travel expenses from Sanofi/Regeneron and Merck & Co. and has a pending patent related to high-affinity T-cell receptors that target the Merkel polyomavirus. SB reports personal fees from Bristol-Myers Squibb, EMD-Serono and personal fees and other from Sanofi-Genzyme; grants from Bristol-Myers Squibb, EMD-Serono, Merck & Co., NantKwest, Novartis, Immune Design, Oncosec, Exicure, Nektar; and personal fees from Castle Biosciences. EJ reports grants from Merck & Co., Bristol-Myers Squibb and Sanofi/Regeneron; personal fees from Bristol-Myers Squibb, Novartis, Array BioPharma, Macrogenics, Sanofi/Regeneron and Genentech. RK reports grants from Merck & Co., Bristol-Myers Squibb and Regeneron; advisory fees from Merck & Co., Bristol-Myers Squibb, Regeneron, Novartis and Array. ASB reports personal fees from Bayer, Deciphera and EMD Serono. BAH reports grants from Merck & Co., Tempest Therapeutics, Olatec Therapeutics, A*STAR Singapore, Sanofi, Leap Therapeutics, GSK and AstraZeneca; personal fees from Merck & Co., Novartis, G1 Therapeutics and CE Concepts; travel fees from ASCO and ASCI and patents related to dendritic cell vaccines, immunotherapy biomarkers and methods for augmenting anti-PD-1 therapy. CC reports a pending patent related to high-affinity T-cell receptors that target the Merkel polyomavirus. JT reports consulting/advisory fees from Merck & Co, Bristol-Myers Squibb, AstraZeneca and Compugen; and a grant from Bristol-Myers Squibb. EJ, MK and BHM are employees of Merck & Co. SPF reports research funding from Merck. MAC reports research funding from Merck & Co. SLT reports that she or an immediate family member has stock and other ownership interests in Aduro Biotech, DNAtrix, Dracen Pharmaceuticals, Dragonfly Therapeutics, Ervaxx, Five Prime Therapeutics, Potenza Therapeutics, RAPT, Tizona Therapeutics, Trieza Therapeutics and WindMIL; a consulting or advisory role in Amgen, DNAtrix, Dragonfly Therapeutics, Dynavax, Ervaxx, Five Prime Therapeutics, Immunocore, Immunomic Therapeutics, Janssen Pharmaceuticals, MedImmune/AstraZeneca, Merck & Co., RAPT and WindMIL; research grants from Bristol-Myers Squibb and Compugen; patents, royalties and/or other intellectual property with Aduro Biotech, Arbor Pharmaceuticals, Bristol-Myers Squibb, Immunomic Therapeutics, NexImmune and WindMIL and travel, accommodations, and expenses from Bristol-Myers Squibb, Dragonfly and Five Prime Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
