IL-17A Secreted by Th17 Cells Is Essential for the Host against Streptococcus agalactiae Infections
- PMID: 33879639
- PMCID: PMC9706036
- DOI: 10.4014/jmb.2103.03053
IL-17A Secreted by Th17 Cells Is Essential for the Host against Streptococcus agalactiae Infections
Abstract
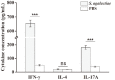
Streptococcus agalactiae is an important bacterial pathogen and causative agent of diseases including neonatal sepsis and meningitis, as well as infections in healthy adults and pregnant women. Although antibiotic treatments effectively relieve symptoms, the emergence and transmission of multidrug-resistant strains indicate the need for an effective immunotherapy. Effector T helper (Th) 17 cells are a relatively newly discovered subpopulation of helper CD4+ T lymphocytes, and which, by expressing interleukin (IL)-17A, play crucial roles in host defenses against a variety of pathogens, including bacteria and viruses. However, whether S. agalactiae infection can induce the differentiation of CD4+ T cells into Th17 cells, and whether IL-17A can play an effective role against S. agalactiae infections, are still unclear. In this study, we analyzed the responses of CD4+ T cells and their defensive effects after S. agalactiae infection. The results showed that S. agalactiae infection induces not only the formation of Th1 cells expressing interferon (IFN)-γ, but also the differentiation of mouse splenic CD4+ T cells into Th17 cells, which highly express IL-17A. In addition, the bacterial load of S. agalactiae was significantly increased and decreased in organs as determined by antibody neutralization and IL-17A addition experiments, respectively. The results confirmed that IL-17A is required by the host to defend against S. agalactiae and that it plays an important role in effectively eliminating S. agalactiae. Our findings therefore prompt us to adopt effective methods to regulate the expression of IL-17A as a potent strategy for the prevention and treatment of S. agalactiae infection.
Keywords: Streptococcus agalactiae; T helper 17 cells; interleukin-17A.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

