[Accuracy of Xpert® MTB/RIF for the detection of tuberculosis and rifampicin-resistance tuberculosis in China: A systematic review and meta-analysis]
- PMID: 33879905
- PMCID: PMC8072426
- DOI: 10.19723/j.issn.1671-167X.2021.02.015
[Accuracy of Xpert® MTB/RIF for the detection of tuberculosis and rifampicin-resistance tuberculosis in China: A systematic review and meta-analysis]
Abstract
Objective: To systematically review the diagnostic accuracy of Xpert® Mycobacterium tuberculosis/rifampicin (Xpert® MTB/RIF) for the detection of active tuberculosis (TB) and rifampicin-resistance TB in Chinese patients.
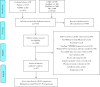
Methods: Four Chinese databases (SinoMed, CNKI, WanFang database, and VIP) and three English databases (PubMed, Embase, and The Cochrane Library) were searched from January 1, 2000 to September 15, 2017, to identify diagnostic tests about the accuracy of Xpert® MTB/RIF in Chinese patients. Two investigators screened the articles and extracted the information independently, and then the quality of each included study was evaluated by Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2. Bivariate random-effects meta-analysis was conducted to pool the sensitivity and specificity. In addition, subgroup analyses were performed based on patient type (TB patient and TB suspected patient), sample type (sputum, bronchoalveolar lavage fluid and others). All statistical analyses were conducted with Stata version 13.0.
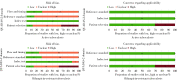
Results: A total of 47 articles were included in this systematic review. Most of them (38 articles) were in Chinese and only 9 articles were in English. All the articles were published during 2014 to 2017, and the sample size ranged from 31 to 3 151. Forty articles including 42 comparisons about TB were finally included with the pooled sensitivity of 0.94 (95%CI: 0.92, 0.95) and the pooled specificity of 0.87 (95%CI: 0.84, 0.91). Subgroup analysis showed that different patient and specimen types had no significant differences on sensitivity, but the specificity of sputum group was higher than that of bronchoalveolar lavage fluid. As for the detection of rifampicin-resistant TB, 33 articles (38 comparisons) were analyzed, the pooled sensitivity and specificity were 0.92 (95%CI: 0.89, 0.94) and 0.98 (95%CI: 0.97, 0.99) respectively. There were no significant differences between the patient and specimen in the subgroup analyses. The Deeks funnel plot showed a possible publication bias for detecting active tuberculosis (P=0.08) and no publication bias for rifampicin-resistant TB (P=0.24). The likelihood ratio scatter gram showed that in clinical applications, Xpert® MTB/RIF had a good diagnostic ability for detecting active tuberculosis, and it had good clinical diagnostic value in detecting rifampicin-resistant TB.
Conclusion: Xpert® MTB/RIF has good sensitivity and specificity in detecting TB and rifampicin-resistant TB in Chinese people. In particular, it has good clinical value in diagnosing rifampicin-resistance TB.
目的: 系统评价结核分枝杆菌/利福平耐药实时荧光定量核酸扩增检测方法(Xpert® Mycobacterium tuberculosis/rifampicin,Xpert® MTB/RIF)对中国人群肺结核(活动性肺结核和利福平耐药肺结核)的诊断准确性。
方法: 计算机检索SinoMed、CNKI、万方、维普、PubMed、Embase、The Cochrane Library数据库,搜集于中国人群中应用Xpert® MTB/RIF方法诊断的研究,检索时间限定为2000年1月1日—2017年9月15日。由2名研究者独立筛选文献、提取资料,使用诊断试验准确性研究的偏倚评估工具(Quality Assessment of Diagnostic Accuracy Studies,QUADAS)-2对纳入的研究进行质量评价。采用Stata 13.0软件进行meta分析,使用双变量随机效应模型合并灵敏度与特异度等诊断准确性指标,根据患者类型和样本类型进行亚组分析。
结果: 最终共纳入47篇文献,其中英文9篇,中文38篇,发表时间为2014—2017年,样本量范围为31~3 151例,活动性肺结核共纳入40篇文献(42个比较组),合并灵敏度为0.94(95%CI: 0.92,0.95),合并特异度为0.87(95%CI: 0.84,0.91)。亚组分析显示,对于不同的样本类型,灵敏度无明显差异,但痰液样本的特异度高于支气管灌洗液样本。利福平耐药肺结核共纳入33篇文献(38个比较组),合并灵敏度为0.92(95%CI: 0.89,0.94),特异度为0.98(95%CI: 0.97,0.99)。Deeks法漏斗图显示,检测活动性肺结核方面可能存在发表偏倚(P=0.08),利福平耐药肺结核方面均不存在发表偏倚(P=0.24)。
结论: 使用Xpert® MTB/RIF方法检测活动性肺结核和利福平耐药肺结核具有较好的灵敏度和特异度,在检测利福平耐药方面具有较好的临床诊断价值。
Keywords: Meta-analysis; Multidrug-resistant tuberculosis; Pulmonary tuberculosis; Xpert® MTB/RIF.
Figures
Similar articles
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Sep 6;9:CD013359. doi: 10.1002/14651858.CD013359.pub3. PMID: 32853411 Free PMC article. Updated.
-
Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms.Cochrane Database Syst Rev. 2021 Mar 23;3(3):CD013694. doi: 10.1002/14651858.CD013694.pub2. Cochrane Database Syst Rev. 2021. PMID: 33755189 Free PMC article.
-
Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD012768. doi: 10.1002/14651858.CD012768.pub3. Cochrane Database Syst Rev. 2021. PMID: 33448348 Free PMC article.
-
Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis.Cochrane Database Syst Rev. 2021 Feb 22;2(2):CD009593. doi: 10.1002/14651858.CD009593.pub5. Cochrane Database Syst Rev. 2021. PMID: 33616229 Free PMC article.
-
Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2019 Jun 7;6(6):CD009593. doi: 10.1002/14651858.CD009593.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 22;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. PMID: 31173647 Free PMC article. Updated.
References
-
-
WHO. Global tuberculosis report 2017[EB/OL]. [2019-01-07]. http://www.who.int/tb/publications/global_report/en/.
-
-
-
WHO. The End TB Strategy[EB/OL]. [2018-12-16]. http://www.who.int/tb/strategy/en/.
-
-
- 陆 震宇, 杨 明芳, 陈 纬. 结核病实验室诊断技术评价及其研究进展. 中国卫生检验杂志. 2017;27(2):301–304.
-
- 刘 宇, 吴 利先. Xpert MTB/RIF在结核病诊断中的研究进展. 中国病原生物学杂志. 2017;12(8):800–802.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials