Vitamin D Levels and Visual System Measurements in Progressive Multiple Sclerosis: A Cross-sectional Study
- PMID: 33880080
- PMCID: PMC8047686
- DOI: 10.7224/1537-2073.2020-005
Vitamin D Levels and Visual System Measurements in Progressive Multiple Sclerosis: A Cross-sectional Study
Abstract
Background: Vitamin D deficiency is associated with increased disease activity in multiple sclerosis (MS), but its role in progressive MS has not been elucidated. The objective was to determine the correlation between vitamin D levels and visual parameters in primary progressive MS (PPMS) and secondary progressive MS (SPMS).
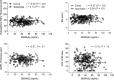
Methods: Serum 25-hydroxyvitamin D (25[OH]D) and 25-hydroxyvitamin D3 (25[OH]D3) levels were obtained from the Secondary and Primary Progressive Ibudilast NeuroNEXT Trial in MS (SPRINT-MS). Visual function measurements and vitamin D associations were determined using the Pearson correlation and the generalized linear mixed model.
Results: The analysis included 258 patients (mean ± SD age of 55.6 ± 7.3 years, 52.7% female, and 52.3% PPMS). Mean vitamin D values were above sufficiency and were similar between PPMS and SPMS (P = .47 and P = .31). There was no association between 25(OH)D3 levels and any visual markers, including peripapillary retinal nerve fiber layer thickness (Spearman r = -0.08), macular volume (r = -0.03), ganglion cell-inner plexiform layer (r = -0.07), and 2.5% low-contrast visual acuity test (r = -0.10). No statistically significant associations between vitamin D levels and visual system measurements were detected in the PPMS and SPMS subgroups.
Conclusions: Vitamin D levels were not associated with optical coherence tomography findings or low-contrast letter acuity in this group of patients with progressive MS.
Keywords: Low-contrast visual acuity; Multiple sclerosis (MS); Optical coherence tomography (OCT); Progressive multiple sclerosis.
© 2021 Consortium of Multiple Sclerosis Centers.
Conflict of interest statement
Financial Disclosures: Dr Fox receives consulting fees from Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic, Novartis, and Teva; serves on advisory committees for Actelion, Biogen, Immunic, and Novartis; and has received clinical trial contract and research grant funding from Biogen and Novartis. Dr Bermel is a consultant for Biogen, Genzyme, Genentech, and Novartis; receives research support from Biogen, Genentech, and Novartis; and shares rights to intellectual property underlying the Multiple Sclerosis Performance Test, currently licensed to Qr8 Health and Biogen. Dr Ontaneda has received grant support from Genzyme, Genentech, and Novartis and consulting fees from Biogen, Genzyme, and Genentech. The other authors declare no conflicts of interest.
Figures
References
-
- Soilu-Hanninen M, Aivo J, Lindstrom BM et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon beta-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–571. - PubMed
-
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. - PubMed
-
- Simpson S, Jr, Taylor B, Blizzard L et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. - PubMed
LinkOut - more resources
Full Text Sources