This is a preprint.
Expression of the ACE2 virus entry protein in the nervus terminalis reveals the potential for an alternative route to brain infection in COVID-19
- PMID: 33880469
- PMCID: PMC8057234
- DOI: 10.1101/2021.04.11.439398
Expression of the ACE2 virus entry protein in the nervus terminalis reveals the potential for an alternative route to brain infection in COVID-19
Update in
-
Expression of the ACE2 Virus Entry Protein in the Nervus Terminalis Reveals the Potential for an Alternative Route to Brain Infection in COVID-19.Front Cell Neurosci. 2021 Jul 5;15:674123. doi: 10.3389/fncel.2021.674123. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34290590 Free PMC article.
Abstract
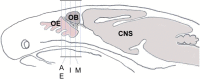
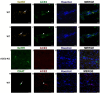
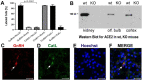
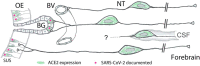
Previous studies suggested that the SARS-CoV-2 virus may gain access to the brain by using a route along the olfactory nerve. However, there is a general consensus that the obligatory virus entry receptor, angiotensin converting enzyme 2 (ACE2), is not expressed in olfactory receptor neurons, and the timing of arrival of the virus in brain targets is inconsistent with a neuronal transfer along olfactory projections. We determined whether nervus terminalis neurons and their peripheral and central projections should be considered as a potential alternative route from the nose to the brain. Nervus terminalis neurons in postnatal mice were double-labeled with antibodies against ACE2 and two nervus terminalis markers, gonadotropin-releasing hormone (GnRH) and choline acetyltransferase (CHAT). We show that a small fraction of CHAT-labeled nervus terminalis neurons, and the large majority of GnRH-labeled nervus terminalis neurons with cell bodies in the region between the olfactory epithelium and the olfactory bulb express ACE2 and cathepsins B and L. Nervus terminalis neurons therefore may provide a direct route for the virus from the nasal epithelium, possibly via innervation of Bowman's glands, to brain targets, including the telencephalon and diencephalon. This possibility needs to be examined in suitable animal models and in human tissues.
Keywords: ACE2; COVID-19; Nervus terminalis; SARS-CoV-2; brain infection; cathepsin; olfactory system.
Figures
Similar articles
-
Expression of the ACE2 Virus Entry Protein in the Nervus Terminalis Reveals the Potential for an Alternative Route to Brain Infection in COVID-19.Front Cell Neurosci. 2021 Jul 5;15:674123. doi: 10.3389/fncel.2021.674123. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34290590 Free PMC article.
-
The olfactory gonadotropin-releasing hormone immunoreactive system in mouse.Brain Res. 1986 Oct 29;386(1-2):351-63. doi: 10.1016/0006-8993(86)90172-1. Brain Res. 1986. PMID: 3535994
-
Primary olfactory projections and the nervus terminalis in the African lungfish: implications for the phylogeny of cranial nerves.Am J Anat. 1988 Aug;182(4):325-34. doi: 10.1002/aja.1001820404. Am J Anat. 1988. PMID: 2847523
-
Function of gonadotropin-releasing hormone in olfaction.Keio J Med. 2001 Jun;50(2):81-5. doi: 10.2302/kjm.50.81. Keio J Med. 2001. PMID: 11450596 Review.
-
The terminal nerve and its relation with extrabulbar "olfactory" projections: lessons from lampreys and lungfishes.Microsc Res Tech. 2004 Sep;65(1-2):13-24. doi: 10.1002/jemt.20095. Microsc Res Tech. 2004. PMID: 15570592 Review.
References
-
- Barnett E.M., and Perlman S. (1993). The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 194(1), 185–191. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
