National trends in prescription drug expenditures and projections for 2021
- PMID: 33880494
- PMCID: PMC8365501
- DOI: 10.1093/ajhp/zxab160
National trends in prescription drug expenditures and projections for 2021
Abstract
Purpose: To report historical patterns of pharmaceutical expenditures, to identify factors that may influence future spending, and to predict growth in drug spending in 2021 in the United States, with a focus on the nonfederal hospital and clinic sectors.
Methods: Historical patterns were assessed by examining data on drug purchases from manufacturers using the IQVIA National Sales Perspectives database. Factors that may influence drug spending in hospitals and clinics in 2021 were reviewed-including new drug approvals, patent expirations, and potential new policies or legislation. Focused analyses were conducted for biosimilars, cancer drugs, generics, coronavirus disease 2019 (COVID-19) pandemic influence, and specialty drugs. For nonfederal hospitals, clinics, and overall (all sectors), estimates of growth of pharmaceutical expenditures in 2021 were based on a combination of quantitative analyses and expert opinion.
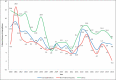
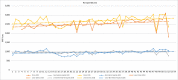
Results: In 2020, overall pharmaceutical expenditures in the United States grew 4.9% compared to 2019, for a total of $535.3 billion. Utilization (a 2.9% increase) and new drugs (a 1.8% increase) drove this increase, with price changes having minimal influence (a 0.3% increase). Adalimumab was the top drug in 2020, followed by apixaban and insulin glargine. Drug expenditures were $35.3 billion (a 4.6% decrease) and $98.4 billion (an 8.1% increase) in nonfederal hospitals and clinics, respectively. In clinics, growth was driven by new products and increased utilization, whereas in hospitals the decrease in expenditures was driven by reduced utilization. Several new drugs that will influence spending are expected to be approved in 2021. Specialty and cancer drugs will continue to drive expenditures along with the evolution of the COVID-19 pandemic.
Conclusion: For 2021, we expect overall prescription drug spending to rise by 4% to 6%, whereas in clinics and hospitals we anticipate increases of 7% to 9% and 3% to 5%, respectively, compared to 2020. These national estimates of future pharmaceutical expenditure growth may not be representative of any particular health system because of the myriad of local factors that influence actual spending.
Keywords: clinics; hospitals; prescription expenditures.
© American Society of Health-System Pharmacists 2021. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Martin AB, Hartman M, Lassman D, Catlin A; National Health Expenditure Accounts Team . National health care spending in 2019: steady growth for the fourth consecutive year. Health Aff (Milwood). 2021;40:14-14. - PubMed
-
- Tichy EM, Schumock GT, Hoffman JM, et al. . National trends in prescription drug expenditures and projections for 2020. Am J Health-Syst Pharm. 2020;77:1213-1230. - PubMed
-
- Feldman WB, Rome BN, Avorn JA, Kesselhaim AS. The future of drug-pricing transparency. N Engl J Med. 384;6:489-491. - PubMed
-
- Center for Drug Evaluation and Research, US Food and Drug Administration. Novel drug approvals for 2020. Current as of January 13, 2021. Accessed February 28, 2021. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and...
-
- US Food and Drug Administration. Biosimilar interchangeable and interchangeable products. Published October 2018. Accessed February 28, 2021. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical