T-Cell Homeostatic Imbalance in Placentas From Women With Human Immunodeficiency Virus in the Absence of Vertical Transmission
- PMID: 33880544
- PMCID: PMC8883807
- DOI: 10.1093/infdis/jiab192
T-Cell Homeostatic Imbalance in Placentas From Women With Human Immunodeficiency Virus in the Absence of Vertical Transmission
Abstract
Background: Implementation of universal antiretroviral therapy (ART) has significantly lowered vertical transmission rates but has also increased numbers of human immunodeficiency virus (HIV)-exposed uninfected children, who remain vulnerable to morbid effects. In the current study, we investigated whether T-cell alterations in the placenta contribute to altered immune status in HIV-exposed uninfected.
Methods: We analyzed T cells from term placenta decidua and villous tissue and paired cord blood from pregnant women living with HIV (PWH) who initiated ART late in pregnancy (n = 21) with pregnant women not living with HIV (PWNH) (n = 9).
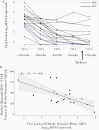
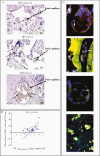
Results: Placentas from PWH showed inverted CD4/CD8 ratios and higher proportions of tissue resident CD8+ T cells in villous tissue relative to control placentas. CD8+ T cells in the fetal capillaries, which were of fetal origin, were positively correlated with maternal plasma viremia before ART initiation, implying that imbalanced T cells persisted throughout pregnancy. In addition, the expanded memory differentiation of CD8+ T cells was confined to the fetal placental compartment and cord blood but was not observed in the maternal decidua.
Conclusions: T-cell homeostatic imbalance in the blood circulation of PWH is reflected in the placenta. The placenta may be a causal link between HIV-induced maternal immune changes during gestation and altered immunity in newborn infants in the absence of vertical transmission.
Keywords: CD4; CD8; HEU; HIV; HIV-exposed; T cells; placenta; placenta pathology; villous tissue.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures




References
-
- Uthman OA, Nachega JB, Anderson J, et al. . Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4:e21–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

