Home Fortification of Complementary Foods Reduces Anemia and Diarrhea among Children Aged 6-18 Months in Bihar, India: A Large-Scale Effectiveness Trial
- PMID: 33880566
- PMCID: PMC8245869
- DOI: 10.1093/jn/nxab065
Home Fortification of Complementary Foods Reduces Anemia and Diarrhea among Children Aged 6-18 Months in Bihar, India: A Large-Scale Effectiveness Trial
Abstract
Background: Home fortification of complementary foods with multiple micronutrient powders (MNPs) is recommended to reduce child anemia in resource-poor settings. However, evidence of program effectiveness in India to guide policies and programs is lacking.
Objectives: We implemented a large-scale intervention of MNPs in Bihar, India. The primary outcome was MNP consumption and change in hemoglobin concentration among children aged 6-18 mo between baseline and endline (12 mo). Secondary outcomes were change in child weight and length and infant and young child feeding (IYCF) practices (initiation, diversity, and feeding frequency). Ad hoc analyses included changes in anemia; stunting; underweight; wasting; and reported diarrhea, fever, and hospitalization.
Methods: We conducted a cluster-randomized, effectiveness trial in >4000 children within the context of ongoing health and nutrition programs implemented by CARE, India. Seventy health subcenters were randomly assigned to receive either MNPs with IYCF counseling (intervention) or IYCF counseling only (control). We used an adjusted difference-in-difference approach using repeat cross-sectional surveys at baseline and endline to evaluate impact.
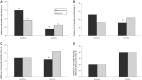
Results: At baseline, 75% of intervention and 69% of control children were anemic and 33% were stunted. By endline, 70% of intervention households reported their child had ever consumed MNPs, and of those, 64% had consumed MNPs in the past month. Relative to control, hemoglobin concentration increased (0.22 g/dL; 95% CI: 0.00, 0.44 g/dL) and anemia declined by 7.1 percentage points (pp) (95% CI: -13.5, -0.7 pp). There was no impact on anthropometry nor IYCF practices. However, there was a decline of 8.0 pp (95% CI: -14.9, -1.1 pp) in stunting among children aged 12-18 mo. Diarrhea prevalence in the past 2 wk was reduced by 4.0 pp (95% CI: -7.6, -0.4 pp).
Conclusions: Home fortification of complementary foods within a government-run program in Bihar had moderate compliance and caused modest improvements in hemoglobin and reductions in anemia and diarrhea prevalence.
Keywords: India; anemia; children; hemoglobin; multiple micronutrient powders.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
Similar articles
-
The Impact of Integrated Infant and Young Child Feeding and Micronutrient Powder Intervention on Feeding Practices and Anemia in Children Aged 6-23 Months in Madagascar.Nutrients. 2017 Jun 7;9(6):581. doi: 10.3390/nu9060581. Nutrients. 2017. PMID: 28590440 Free PMC article.
-
An Integrated Infant and Young Child Feeding and Micronutrient Powder Intervention Does Not Affect Anemia, Iron Status, or Vitamin A Status among Children Aged 12-23 Months in Eastern Uganda.J Nutr. 2020 Apr 1;150(4):938-944. doi: 10.1093/jn/nxz314. J Nutr. 2020. PMID: 31923315 Free PMC article.
-
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age.Cochrane Database Syst Rev. 2020 Feb 28;2(2):CD008959. doi: 10.1002/14651858.CD008959.pub3. Cochrane Database Syst Rev. 2020. PMID: 32107773 Free PMC article.
-
Effectiveness of a home fortification programme with multiple micronutrients on infant and young child development: a cluster-randomised trial in rural Bihar, India.Br J Nutr. 2018 Jul;120(2):176-187. doi: 10.1017/S000711451800140X. Br J Nutr. 2018. PMID: 29947323 Free PMC article. Clinical Trial.
-
Infant and Young Child Feeding (IYCF) Practices Improved in 2 Districts in Nepal during the Scale-Up of an Integrated IYCF and Micronutrient Powder Program.Curr Dev Nutr. 2018 Apr 25;2(6):nzy019. doi: 10.1093/cdn/nzy019. eCollection 2018 Jun. Curr Dev Nutr. 2018. PMID: 29984348 Free PMC article. Review.
Cited by
-
Community-Based Child Food Interventions/Supplements for the Prevention of Wasting in Children Up to 5 Years at Risk of Wasting and Nutritional Oedema: A Systematic Review and Meta-Analysis.Nutr Rev. 2025 Aug 1;83(8):1402-1424. doi: 10.1093/nutrit/nuaf041. Nutr Rev. 2025. PMID: 40272950 Free PMC article.
-
Child-Owned Poultry Intervention Effects on Hemoglobin, Anemia, Concurrent Anemia and Stunting, and Morbidity Status of Young Children in Southern Ethiopia: A Cluster Randomized Controlled Community Trial.Int J Environ Res Public Health. 2023 Apr 5;20(7):5406. doi: 10.3390/ijerph20075406. Int J Environ Res Public Health. 2023. PMID: 37048019 Free PMC article. Clinical Trial.
-
The effect of interventions distributing home fortification products on infant and young child feeding (IYCF) practices: A systematic narrative review.Matern Child Nutr. 2023 Jul;19(3):e13488. doi: 10.1111/mcn.13488. Epub 2023 Feb 26. Matern Child Nutr. 2023. PMID: 36842164 Free PMC article.
References
-
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE;the Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group . Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet North Am Ed. 2013;382(9890):452–77. - PubMed
-
- WHO . Global strategy for women's, children's and adolescents’ health (2016–2030). Geneva (Switzerland): WHO; 2016. [Internet]. [Accessed 2020 Aug 1]. Available from: https://www.who.int/life-course/partners/global-strategy/global-strategy....
-
- WHO . Guideline: use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva (Switzerland): WHO; 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

