Functional compartmentalization of photoreceptor neurons
- PMID: 33880652
- PMCID: PMC8690575
- DOI: 10.1007/s00424-021-02558-7
Functional compartmentalization of photoreceptor neurons
Abstract
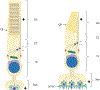
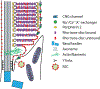
Retinal photoreceptors are neurons that convert dynamically changing patterns of light into electrical signals that are processed by retinal interneurons and ultimately transmitted to vision centers in the brain. They represent the essential first step in seeing without which the remainder of the visual system is rendered moot. To support this role, the major functions of photoreceptors are segregated into three main specialized compartments-the outer segment, the inner segment, and the pre-synaptic terminal. This compartmentalization is crucial for photoreceptor function-disruption leads to devastating blinding diseases for which therapies remain elusive. In this review, we examine the current understanding of the molecular and physical mechanisms underlying photoreceptor functional compartmentalization and highlight areas where significant knowledge gaps remain.
Keywords: Arrestin; Cilia; Membrane proteins; Photoreceptor; Rhodopsin; Trafficking.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures



References
-
- Ames JB, et al. , Amino-terminal myristoylation induces cooperative calcium binding to recoverin. J Biol Chem, 1995. 270(9): p. 4526–33. - PubMed
-
- Ames JB, et al. , Nuclear magnetic resonance evidence for Ca(2+)-induced extrusion of the myristoyl group of recoverin. J Biol Chem, 1995. 270(52): p. 30909–13. - PubMed
-
- Anant JS, et al. , In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem, 1992. 267(2): p. 687–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

