NMR Analysis of Carboxylate Isotopomers of 13C-Metabolites by Chemoselective Derivatization with 15N-Cholamine
- PMID: 33880916
- PMCID: PMC9350721
- DOI: 10.1021/acs.analchem.0c04220
NMR Analysis of Carboxylate Isotopomers of 13C-Metabolites by Chemoselective Derivatization with 15N-Cholamine
Abstract
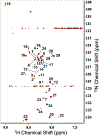
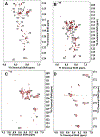
A substantial fraction of common metabolites contains carboxyl functional groups. Their 13C isotopomer analysis by nuclear magnetic resonance (NMR) is hampered by the low sensitivity of the 13C nucleus, the slow longitudinal relaxation for the lack of an attached proton, and the relatively low chemical shift dispersion of carboxylates. Chemoselective (CS) derivatization is a means of tagging compounds in a complex mixture via a specific functional group. 15N1-cholamine has been shown to be a useful CS agent for carboxylates, producing a peptide bond that can be detected via 15N-attached H with high sensitivity in heteronuclear single quantum coherence experiments. Here, we report an improved method of derivatization and show how 13C-enrichment at the carboxylate and/or the adjacent carbon can be determined via one- and two-bond coupling of the carbons adjacent to the cholamine 15N atom in the derivatives. We have applied this method for the determination of 13C isotopomer distribution in the extracts of A549 cell culture and liver tissue from a patient-derived xenograft mouse.
Figures


Similar articles
-
Synthesis of [15 N]-cholamine bromide hydrobromide.J Labelled Comp Radiopharm. 2018 Apr;61(4):391-394. doi: 10.1002/jlcr.3598. Epub 2018 Mar 30. J Labelled Comp Radiopharm. 2018. PMID: 29278650 Free PMC article.
-
15N-cholamine--a smart isotope tag for combining NMR- and MS-based metabolite profiling.Anal Chem. 2013 Sep 17;85(18):8715-21. doi: 10.1021/ac401712a. Epub 2013 Aug 26. Anal Chem. 2013. PMID: 23930664 Free PMC article.
-
A Unique Collision-Induced Dissociation Reaction of Cholamine Derivatives of Certain Prostaglandins.J Am Soc Mass Spectrom. 2018 Dec;29(12):2360-2367. doi: 10.1007/s13361-018-2051-6. Epub 2018 Aug 27. J Am Soc Mass Spectrom. 2018. PMID: 30151680
-
Emerging new strategies for successful metabolite identification in metabolomics.Bioanalysis. 2016 Mar;8(6):557-73. doi: 10.4155/bio-2015-0004. Epub 2016 Feb 26. Bioanalysis. 2016. PMID: 26915807 Free PMC article. Review.
-
Review of 13carbon nuclear magnetic resonance characterizations of dimethyltin carboxylates.Heliyon. 2022 Sep 8;8(9):e10507. doi: 10.1016/j.heliyon.2022.e10507. eCollection 2022 Sep. Heliyon. 2022. PMID: 36148282 Free PMC article. Review.
Cited by
-
Isobaric 4-Plex Tagging for Absolute Quantitation of Biological Acids in Diabetic Urine Using Capillary LC-MS/MS.ACS Meas Sci Au. 2022 Jun 15;2(3):287-295. doi: 10.1021/acsmeasuresciau.1c00061. Epub 2022 Mar 3. ACS Meas Sci Au. 2022. PMID: 35726255 Free PMC article.
-
NMR-Based Stable Isotope Tracing of Cancer Metabolism.Methods Mol Biol. 2025;2855:457-504. doi: 10.1007/978-1-0716-4116-3_26. Methods Mol Biol. 2025. PMID: 39354323
-
Neutron encoded derivatization of endothelial cell lysates for quantitation of aldehyde metabolites using nESI-LC-HRMS.Anal Chim Acta. 2022 Jan 15;1190:339260. doi: 10.1016/j.aca.2021.339260. Epub 2021 Nov 9. Anal Chim Acta. 2022. PMID: 34857138 Free PMC article.
-
NMR-based isotope editing, chemoselection and isotopomer distribution analysis in stable isotope resolved metabolomics.Methods. 2022 Oct;206:8-17. doi: 10.1016/j.ymeth.2022.07.014. Epub 2022 Jul 28. Methods. 2022. PMID: 35908585 Free PMC article. Review.
References
-
- Nicholson JK; Holmes E; Wilson ID (2005). "Gut microorganisms, mammalian metabolism and personalized health care." Nature reviews. Microbiology 3(5): 431–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

