Illuminating Life's Origins: UV Photochemistry in Abiotic Synthesis of Biomolecules
- PMID: 33880920
- PMCID: PMC8240947
- DOI: 10.1021/jacs.1c01839
Illuminating Life's Origins: UV Photochemistry in Abiotic Synthesis of Biomolecules
Abstract
Solar radiation is the principal source of energy available to Earth and has unmatched potential for the synthesis of organic material from primordial molecular building blocks. As well as providing the energy for photochemical synthesis of (proto)biomolecules of interest in origins of life-related research, light has also been found to often provide remarkable selectivity in these processes, for molecules that function in extant biology and against those that do not. As such, light is heavily implicated as an environmental input on the nascent Earth that was important for the emergence of complex yet selective chemical systems underpinning life. Reactivity and selectivity in photochemical prebiotic synthesis are discussed, as are their implications for origins of life scenarios and their plausibility, and the future directions of this research.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
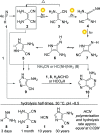
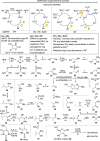
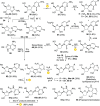
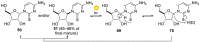
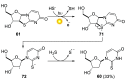
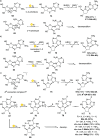
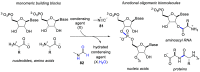
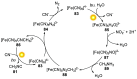
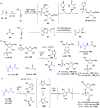

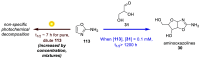
References
-
- Sutherland J. D. Opinion: Studies on the Origin of Life — the End of the Beginning. Nat. Rev. Chem. 2017, 1 (2), 0012. 10.1038/s41570-016-0012. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

