Clinical Characterization of Mogamulizumab-Associated Rash During Treatment of Mycosis Fungoides or Sézary Syndrome
- PMID: 33881447
- PMCID: PMC8060888
- DOI: 10.1001/jamadermatol.2021.0877
Clinical Characterization of Mogamulizumab-Associated Rash During Treatment of Mycosis Fungoides or Sézary Syndrome
Abstract
Importance: Mogamulizumab is a monoclonal antibody against CCR4 approved for treatment for mycosis fungoides (MF) and Sézary syndrome (SS). Mogamulizumab-associated rash (MAR) is difficult to differentiate from cutaneous MF or SS, which can lead to unnecessary discontinuation of drug use because of concern for severe drug reaction or incorrect presumption of disease relapse or progression in the skin.
Objective: To examine the most common clinical presentations of MAR in patients with MF or SS and the diagnostic and management challenges.
Design, setting, and participants: This retrospective case series assessed patients from a multidisciplinary cutaneous lymphoma clinic and supportive oncodermatology clinic at a major academic referral center who had a diagnosis of MF or SS and received mogamulizumab from January 1, 2013, to January 1, 2020. Treatment was followed by new or worsening rash with skin biopsy results compatible with drug eruption determined by clinicopathologic correlation and molecular testing to exclude active malignant disease.
Exposures: At least 1 dose of mogamulizumab.
Main outcomes and measures: Mogamulizumab-associated rash was characterized by clinical features, including time to onset, clinical presentation, histopathologic features, and management approach.
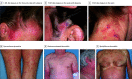
Results: The study included 19 patients with MF or SS who developed MAR (median age, 65 years; age range, 38-82 years; 10 [52.6%] male). Median time to MAR onset was 119 days (range, 56 days to 3.8 years). Patients with MAR exhibited 4 predominant clinical presentations: (1) folliculotropic MF-like scalp plaques with alopecia, (2) papules and/or plaques, (3) photoaccentuated dermatitis, and (4) morbilliform or erythrodermic dermatitis. The most common anatomical region involved was the head and neck, including the scalp. Histopathologic findings were variable and did not correspond to primary clinical morphologic findings. Immunohistochemistry and T-cell clonality ancillary testing were helpful to distinguish MAR from disease. Most patients with MAR (14 of 19) discontinued mogamulizumab treatment; however, no life-threatening severe cutaneous adverse drug reactions occurred, and the decision for drug therapy cessation was usually multifactorial. Four patients were treated again with mogamulizumab with no life-threatening drug-related events. Approaches to management of MAR include topical corticosteroids, systemic corticosteroids, and/or methotrexate.
Conclusions and relevance: This case series found that mogamulizumab-associated rash had a heterogeneous clinical presentation with variable and delayed onset in patients with MF or SS. Mogamulizumab-associated rash exhibited a predilection for the head and neck and was difficult to clinically distinguish from relapse or progression of disease. Recognition of the most common clinical presentations can help prevent unnecessary discontinuation of mogamulizumab treatment. The presence of MAR does not necessitate permanent discontinuation of or avoidance of retreatment with mogamulizumab.
Conflict of interest statement
Figures

References
-
- Kim YH, Bagot M, Pinter-Brown L, et al. ; MAVORIC Investigators . Mogamulizumab Versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. doi:10.1016/S1470-2045(18)30379-6 - DOI - PubMed
-
- Ni X, Jorgensen JL, Goswami M, et al. . Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2015;21(2):274-285. doi:10.1158/1078-0432.CCR-14-0830 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

