Cardiovascular Biomarkers in the Early Discrimination of Type 2 Myocardial Infarction
- PMID: 33881449
- PMCID: PMC8060883
- DOI: 10.1001/jamacardio.2021.0669
Cardiovascular Biomarkers in the Early Discrimination of Type 2 Myocardial Infarction
Abstract
Importance: Rapid and accurate noninvasive discrimination of type 2 myocardial infarction (T2MI), which is because of a supply-demand mismatch, from type 1 myocardial infarction (T1MI), which arises via plaque rupture, is essential, because treatment differs substantially. Unfortunately, this is a major unmet clinical need, because even high-sensitivity cardiac troponin (hs-cTn) measurement provides only modest accuracy.
Objective: To test the hypothesis that novel cardiovascular biomarkers quantifying different pathophysiological pathways involved in T2MI and/or T1MI may aid physicians in the rapid discrimination of T2MI vs T1MI.
Design, setting, and participants: This international, multicenter prospective diagnostic study was conducted in 12 emergency departments in 5 countries (Switzerland, Spain, Italy, Poland, and the Czech Republic) with patients presenting with acute chest discomfort to the emergency departments. The study quantified the discrimination of hs-cTn T, hs-cTn I, and 17 novel cardiovascular biomarkers measured in subsets of consecutively enrolled patients against a reference standard (final diagnosis), centrally adjudicated by 2 independent cardiologists according to the fourth universal definition of MI, using all information, including cardiac imaging and serial measurements of hs-cTnT or hs-cTnI.
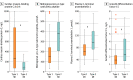
Results: Among 5887 patients, 1106 (18.8%) had an adjudicated final diagnosis of MI; of these, 860 patients (77.8%) had T1MI, and 246 patients (22.2%) had T2MI. Patients with T2MI vs those with T1MI had lower concentrations of biomarkers quantifying cardiomyocyte injury hs-cTnT (median [interquartile range (IQR)], 30 (17-55) ng/L vs 58 (28-150) ng/L), hs-cTnI (median [IQR], 23 [10-83] ng/L vs 115 [28-576] ng/L; P < .001), and cardiac myosin-binding protein C (at presentation: median [IQR], 76 [38-189] ng/L vs 257 [75-876] ng/L; P < .001) but higher concentrations of biomarkers quantifying endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress (median [IQR] values: C-terminal proendothelin 1, 97 [75-134] pmol/L vs 68 [55-91] pmol/L; midregional proadrenomedullin, 0.97 [0.67-1.51] pmol/L vs 0.72 [0.53-0.99] pmol/L; midregional pro-A-type natriuretic peptide, 378 [207-491] pmol/L vs 152 [90-247] pmol/L; and growth differentiation factor 15, 2.26 [1.44-4.35] vs 1.56 [1.02-2.19] ng/L; all P < .001). Discrimination for these biomarkers, as quantified by the area under the receiver operating characteristics curve, was modest (hs-cTnT, 0.67 [95% CI, 0.64-0.71]; hs-cTn I, 0.71 [95% CI, 0.67-0.74]; cardiac myosin-binding protein C, 0.67 [95% CI, 0.61-0.73]; C-terminal proendothelin 1, 0.73 [95% CI, 0.63-0.83]; midregional proadrenomedullin, 0.66 [95% CI, 0.60-0.73]; midregional pro-A-type natriuretic peptide, 0.77 [95% CI, 0.68-0.87]; and growth differentiation factor 15, 0.68 [95% CI, 0.58-0.79]).
Conclusions and relevance: In this study, biomarkers quantifying myocardial injury, endothelial dysfunction, microvascular dysfunction, and/or hemodynamic stress provided modest discrimination in early, noninvasive diagnosis of T2MI.
Conflict of interest statement
Figures

Comment in
-
Differentiating Type 1 and Type 2 Myocardial Infarction: Unfortunately, Still More Art Than Science.JAMA Cardiol. 2021 Jul 1;6(7):781. doi: 10.1001/jamacardio.2021.0693. JAMA Cardiol. 2021. PMID: 33881457 No abstract available.
-
Biomarkers for Myocardial Infarction Type Discrimination-The Key Might Be in the Time Course of the Disease-Reply.JAMA Cardiol. 2022 Jan 1;7(1):113-114. doi: 10.1001/jamacardio.2021.4395. JAMA Cardiol. 2022. PMID: 34705018 No abstract available.
-
Biomarkers for Myocardial Infarction Type Discrimination-The Key Might Be in the Time Course of the Disease.JAMA Cardiol. 2022 Jan 1;7(1):112-113. doi: 10.1001/jamacardio.2021.4398. JAMA Cardiol. 2022. PMID: 34705019 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

