Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis
- PMID: 33881478
- PMCID: PMC8060891
- DOI: 10.1001/jamasurg.2021.0891
Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis
Abstract
Importance: An overview of rates of axillary pathologic complete response (pCR) for all breast cancer subtypes, both for patients with and without pathologically proven clinically node-positive disease, is lacking.
Objective: To provide pooled data of all studies in the neoadjuvant setting on axillary pCR rates for different breast cancer subtypes in patients with initially clinically node-positive disease.
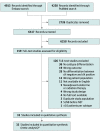
Data sources: The electronic databases Embase and PubMed were used to conduct a systematic literature search on July 16, 2020. The references of the included studies were manually checked to identify other eligible studies.
Study selection: Studies in the neoadjuvant therapy setting were identified regarding axillary pCR for different breast cancer subtypes in patients with initially clinically node-positive disease (ie, defined as node-positive before the initiation of neoadjuvant systemic therapy).
Data extraction and synthesis: Two reviewers independently selected eligible studies according to the inclusion criteria and extracted all data. All discrepant results were resolved during a consensus meeting. To identify the different subtypes, the subtype definitions as reported by the included articles were used. The random-effects model was used to calculate the overall pooled estimate of axillary pCR for each breast cancer subtype.
Main outcomes and measures: The main outcome of this study was the rate of axillary pCR and residual axillary lymph node disease after neoadjuvant systemic therapy for different breast cancer subtypes, differentiating studies with and without patients with pathologically proven clinically node-positive disease.
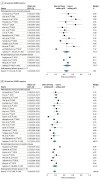
Results: This pooled analysis included 33 unique studies with 57 531 unique patients and showed the following axillary pCR rates for each of the 7 reported subtypes in decreasing order: 60% for hormone receptor (HR)-negative/ERBB2 (formerly HER2)-positive, 59% for ERBB2-positive (HR-negative or HR-positive), 48% for triple-negative, 45% for HR-positive/ERBB2-positive, 35% for luminal B, 18% for HR-positive/ERBB2-negative, and 13% for luminal A breast cancer. No major differences were found in the axillary pCR rates per subtype by analyzing separately the studies of patients with and without pathologically proven clinically node-positive disease before neoadjuvant systemic therapy.
Conclusions and relevance: The HR-negative/ERBB2-positive subtype was associated with the highest axillary pCR rate. These data may help estimate axillary treatment response in the neoadjuvant setting and thus select patients for more or less invasive axillary procedures.
Conflict of interest statement
Figures


References
-
- Rouzier R, Extra JM, Klijanienko J, et al. . Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20(5):1304-1310. doi:10.1200/JCO.2002.20.5.1304 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

