Self-Organized Amphiphiles Are Poor Hydroxyl Radical Scavengers in Fast Photochemical Oxidation of Proteins Experiments
- PMID: 33881849
- PMCID: PMC8790760
- DOI: 10.1021/jasms.0c00457
Self-Organized Amphiphiles Are Poor Hydroxyl Radical Scavengers in Fast Photochemical Oxidation of Proteins Experiments
Abstract
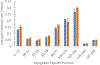
Analysis of membrane protein topography using fast photochemical oxidation of proteins (FPOP) has been reported in recent years but is still underrepresented in literature. Based on the hydroxyl radical reactivity of lipids and other amphiphiles, it is believed that the membrane environment acts as a hydroxyl radical scavenger decreasing effective hydroxyl radical doses and resulting in less observed oxidation of proteins. We found no significant change in bulk solvent radical scavenging activity upon the addition of disrupted cellular membranes up to 25600 cells/μL using an inline radical dosimeter. We confirmed the nonscavenging nature of the membrane in bulk solution with the FPOP results of a soluble model protein in the presence of cell membranes, which showed no significant difference in oxidation with or without membranes. The use of detergents revealed that, while soluble detergent below the critical micelle concentration is a potent hydroxyl radical scavenger, additional detergent has little to no hydroxyl radical scavenging effect once the critical micelle concentration is reached. Examination of both an extracellular peptide of the integral membrane protein bacteriorhodopsin as well as a novel hydroxyl radical dosimeter tethered to a Triton X-series amphiphile indicate that proximity to the membrane surface greatly decreases reaction with hydroxyl radicals, even though the oxidation target is equally solvent accessible. These results suggest that the observed reduced oxidation of solvent-accessible surfaces of integral membrane proteins is due to the high local concentration of radical scavengers in the membrane or membrane mimetics competing for the local concentration of hydroxyl radicals.
Keywords: FPOP; covalent labeling; hydroxyl radical protein footprinting; membrane proteins; structural biology.
Conflict of interest statement
Conflict of Interest
J.S.S. discloses a significant financial interest in GenNext Technologies, Inc., a small company seeking to commercialize technologies for protein higher-order structure analysis.
Figures

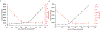
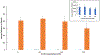
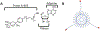
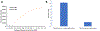
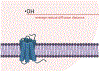
References
-
- Filmore D It’s a GPCR world. Modern drug discovery. 7(24–28 (2004)
-
- Drews J Drug Discovery: A historical prospective. Science. 287(5460), 1960–1964 (2000) - PubMed
-
- Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M Drug-target network. Nat Biotechnol. 25(10), 1119–1126 (2007) - PubMed
-
- Khanal A, Pan Y, Brown LS, Konermann L Pulsed hydrogen/deuterium exchange mass spectrometry for time-resolved membrane protein folding studies. J Mass Spectrom. 47(12), 1620–1626 (2012) - PubMed
-
- Pan Y, Brown L, Konermann L Hydrogen/deuterium exchange mass spectrometry and optical spectroscopy as complementary tools for studying the structure and dynamics of a membrane protein. International Journal of Mass Spectrometry. 302(1–3), 3–11 (2011)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

